Biochemistry: Nucleic Acids Exam
Test your knowledge with these 40 questions.
Nucleic Acids Exam
Question 1/40
Exam Complete!
Here are your results, .
Your Score
38/40
95%
Test your knowledge with these 40 questions.
Question 1/40
Here are your results, .
Your Score
38/40
95%
Test your knowledge with these 30 questions.
Question 1/30
Here are your results, .
Your Score
28/30
93%
Proteins are undoubtedly the most versatile and functionally diverse macromolecules in living systems. They are massive, complex organic compounds that are absolutely essential for every living cell, performing the vast majority of biological tasks. Indeed, if you can imagine a job that needs doing in a cell, chances are a protein is doing it.
Think of them as the true "workhorses" of the cell. While carbohydrates are primarily for immediate energy and structural components, and lipids for membranes and long-term energy storage, proteins execute an astonishing array of functions, making life possible and dynamic.
The word "protein" is derived from the Greek word "proteios."
This etymology beautifully underscores their profound significance: proteins are indeed of utmost importance to life, playing a primary and central role in virtually every biological process, from molecular interactions to macroscopic tissue function.
While carbohydrates and lipids primarily consist of carbon, hydrogen, and oxygen, proteins possess a broader and more distinctive elemental signature:
Proteins are truly the "workhorses" that carry out the cellular instructions and enable all aspects of life. Their functions are incredibly diverse and sophisticated:
Proteins provide the framework and strength for cells and tissues. Examples include Collagen (in skin, bone), Elastin (in blood vessels), Keratin (in hair, nails), and Actin/Tubulin (in the cytoskeleton).
As enzymes, proteins speed up nearly all biochemical reactions. Examples include Amylase (digests starch) and DNA Polymerase (synthesizes DNA). Deficiencies can cause metabolic diseases.
Proteins move essential molecules. Hemoglobin transports oxygen, Albumin transports fatty acids and drugs, Lipoproteins transport fats, and Transferrin transports iron. Ferritin stores iron inside cells.
Contractile proteins enable all forms of biological movement. Actin and Myosin power muscle contraction, while Dynein and Kinesin move cargo within cells and power cilia and flagella.
Proteins regulate physiological processes. Examples include protein hormones like Insulin, cell surface Receptors that transmit signals, and Transcription Factors that control gene expression.
Proteins protect the body from pathogens. Antibodies (Immunoglobulins) recognize and neutralize foreign invaders, while Cytokines and Complement proteins coordinate the immune response.
Plasma proteins like Albumin maintain osmotic pressure, preventing tissue edema. Coagulation factors like Fibrinogen and Thrombin are essential for blood clotting and preventing blood loss after injury.
While not their primary function, proteins can be broken down into amino acids and used for energy during times of starvation or when other energy stores are depleted, through processes like gluconeogenesis.
Remember the functional group Amino? Indeed, it's central to these vital molecules!
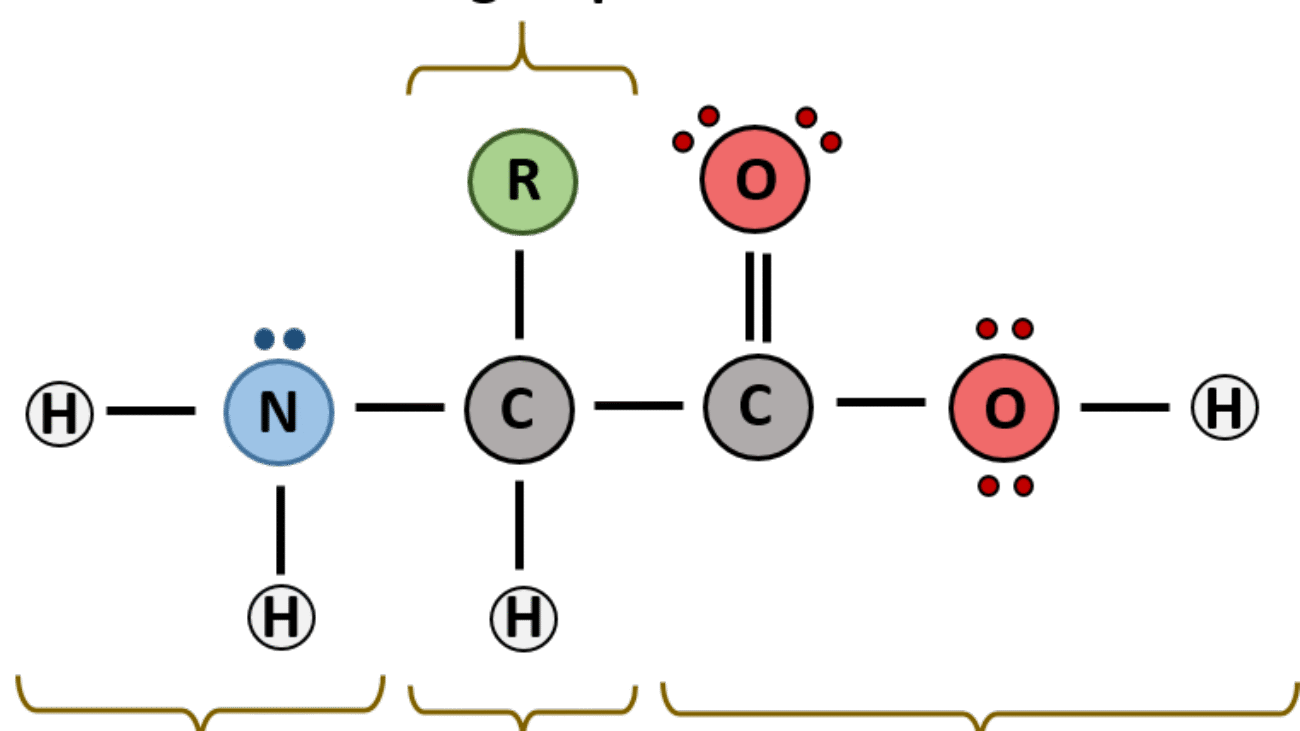
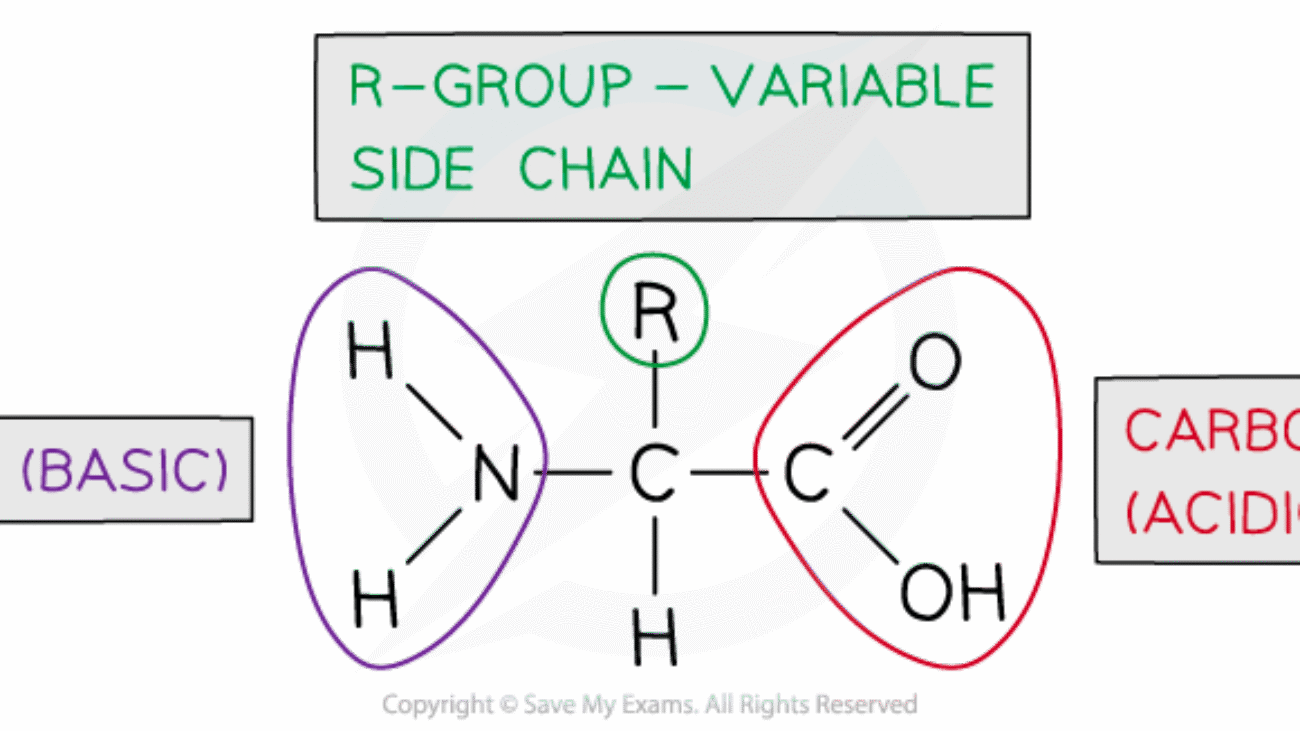
An amino acid is an organic molecule characterized by its unique chemical structure: it features a central carbon atom (the α-carbon) covalently bonded to four distinct groups:
The term amino acid is short for α-amino carboxylic acid, emphasizing the attachment of both the amino and carboxyl groups to the same carbon atom (the α-carbon).
Every single one of the 20 common genetically encoded amino acids shares a very similar basic blueprint:
At the normal pH inside the body (physiological pH, ~7.4), the amino group typically carries a positive charge (NH3+), and the carboxyl group carries a negative charge (COO−). This means that a single amino acid, even with both positive and negative parts, can have an overall neutral charge. When a molecule possesses both a positive and a negative charge, it's called a zwitterion.
To build the long, complex chains of proteins, we need individual building blocks. These single parts are called monomers. In this case, amino acids are the fundamental monomers. When many amino acid monomers come together and link chemically, they form polymers, which are the proteins.
In simple terms: Amino acids are the building blocks, and proteins are the intricate structures built from these blocks.
Amino acids are the fundamental monomer units that link together to form polypeptides, which then fold into functional proteins. There are 20 common amino acids that are genetically encoded and found in most proteins, although many other non-proteinogenic amino acids exist in nature (e.g., modified amino acids, neurotransmitters like GABA).
A peptide bond is formed through a condensation reaction (also known as dehydration synthesis).
A peptide bond can be broken through a reaction called hydrolysis.
When amino acids link together to form a peptide, they lose some atoms (the elements of water) in the process. The amino acid that has been incorporated into the chain, now missing those elements, is no longer a "free amino acid" with its full amino and carboxyl groups. Instead, it's now a "leftover part" or a "component" of the larger chain. For this reason:
R
|
H₂N − C − COOH (General form - often drawn this way for simplicity,
| but not how it primarily exists in solution)
H
At highly acidic pH (low pH, excess H+):
R
|
+H₃N − CH − COOH (Cationic form at low pH)
At physiological pH (neutral pH, ~7.4):
R
|
+H₃N − CH − COO− (Zwitterion or dipolar ion at physiological pH)
At highly basic pH (high pH, low H+):
R
|
H₂N − CH − COO− (Anionic form at high pH)
As we discussed, the "Side Chain" or "R-Group" is the only part that varies among the 20 common amino acids found in proteins. These R-groups have different chemical properties that dictate the amino acid's behavior and, consequently, the protein's overall structure and function.
We can classify these 20 amino acids into several groups based on the polarity and charge of their R-groups at physiological pH (around 7.4).
These R-groups are generally "water-fearing" (hydrophobic) because they consist mainly of hydrocarbons (carbon and hydrogen atoms), which do not readily form hydrogen bonds with water. They tend to cluster together in the interior of proteins, away from the aqueous environment.
| Name | 3-Letter | 1-Letter | Structure of R-Group | Key Characteristics |
|---|---|---|---|---|
| Glycine | Gly | G | -H (just a hydrogen atom) | Smallest & simplest. Only non-chiral amino acid. Allows for great flexibility in protein structure due to its small size. |
| Alanine | Ala | A | -CH₃ (methyl group) | Small, unreactive. Contributes to the hydrophobic core of proteins. |
| Valine | Val | V | -CH(CH₃)₂ (isopropyl group) | Branched hydrocarbon chain. More hydrophobic than Alanine. |
| Leucine | Leu | L | -CH₂CH(CH₃)₂ (isobutyl group) | Branched hydrocarbon chain. Very hydrophobic. Common in the interior of proteins. |
| Isoleucine | Ile | I | -CH(CH₃)CH₂CH₃ (sec-butyl group) | Branched hydrocarbon chain. Stereoisomer of Leucine (same atoms, different arrangement). Very hydrophobic. |
| Methionine | Met | M | -CH₂CH₂SCH₃ (contains a sulfur atom) | Contains a sulfur atom (thioether linkage), but it's largely nonpolar. Always the first amino acid in a newly synthesized polypeptide chain (start codon). |
| Proline | Pro | P | -CH₂CH₂CH₂- (cyclic structure) | Unique cyclic structure where its R-group is bonded to both the α-carbon and the α-amino group, forming a rigid ring. Causes "kinks" in polypeptide chains. Often found in turns. |
These R-groups contain bulky ring structures, which makes them generally hydrophobic. They can also absorb UV light at 280 nm, a property used to quantify proteins.
| Name | 3-Letter | 1-Letter | Structure of R-Group | Key Characteristics |
|---|---|---|---|---|
| Phenylalanine | Phe | F | -CH₂- (phenyl group) | Very hydrophobic due to the bulky phenyl ring. |
| Tyrosine | Tyr | Y | -CH₂- (phenyl group with -OH) | Aromatic ring with a hydroxyl (-OH) group. The -OH group can form hydrogen bonds, making it slightly more polar than Phenylalanine. Can be phosphorylated, important for cell signaling. |
| Tryptophan | Trp | W | -CH₂- (indole group, double ring with N) | Largest and most hydrophobic aromatic amino acid. Indole ring can form hydrogen bonds through its N-H group. Precursor to serotonin and niacin. |
These R-groups contain functional groups that can form hydrogen bonds with water (like -OH, -SH, -CONH₂), making them "water-loving" (hydrophilic). They tend to be found on the surface of proteins, interacting with the aqueous environment.
| Name | 3-Letter | 1-Letter | Structure of R-Group | Key Characteristics |
|---|---|---|---|---|
| Serine | Ser | S | -CH₂OH (hydroxyl group) | Contains a hydroxyl group. Can form hydrogen bonds. Can be phosphorylated, important for cell signaling. |
| Threonine | Thr | T | -CH(OH)CH₃ (hydroxyl group) | Contains a hydroxyl group. Can form hydrogen bonds. Can be phosphorylated. |
| Cysteine | Cys | C | -CH₂SH (sulfhydryl group) | Contains a sulfhydryl (-SH) group. Crucially, two Cysteine residues can form a disulfide bond (-S-S-), a strong covalent bond that stabilizes protein structure. |
| Asparagine | Asn | N | -CH₂CONH₂ (amide group) | Contains an amide group. Can form hydrogen bonds. |
| Glutamine | Gln | Q | -CH₂CH₂CONH₂ (amide group) | Contains an amide group. Can form hydrogen bonds. Longer side chain than Asparagine. |
These R-groups contain an extra amino group or other nitrogen-containing groups that can accept a proton (H⁺) at physiological pH, making them positively charged (basic). They are very hydrophilic and are usually found on the surface of proteins.
| Name | 3-Letter | 1-Letter | Structure of R-Group | Key Characteristics |
|---|---|---|---|---|
| Lysine | Lys | K | -CH₂CH₂CH₂CH₂NH₃⁺ (primary amine) | Long hydrocarbon chain with a terminal primary amino group. Strongly basic and positively charged at neutral pH. |
| Arginine | Arg | R | -CH₂CH₂CH₂NHC(=NH)NH₂⁺ (guanidinium group) | Contains a guanidinium group, which is the most strongly basic functional group in amino acids. Always positively charged at neutral pH. |
| Histidine | His | H | -CH₂- (imidazole group) | Contains an imidazole ring. Unique in that its side chain can be either uncharged or positively charged at physiological pH (pKa near 6.0). This makes it important in enzyme active sites, where it can act as both a proton donor and acceptor. |
These R-groups contain an extra carboxyl group that can donate a proton (H⁺) at physiological pH, making them negatively charged (acidic). They are very hydrophilic and are usually found on the surface of proteins, often involved in ionic interactions.
| Name | 3-Letter | 1-Letter | Structure of R-Group | Key Characteristics |
|---|---|---|---|---|
| Aspartate | Asp | D | -CH₂COO⁻ (carboxylic acid group) | Contains a second carboxyl group. Negatively charged at neutral pH. Often participates in ionic bonds and salt bridges. |
| Glutamate | Glu | E | -CH₂CH₂COO⁻ (carboxylic acid group) | Contains a second carboxyl group. Negatively charged at neutral pH. Longer side chain than Aspartate. |
A peptide or protein sequence is the specific linear order of amino acids linked together by peptide bonds. There are very specific conventions for how these sequences are written and read, which are essential for clear, unambiguous communication in biochemistry and molecular biology.
Every peptide or polypeptide chain exhibits a distinct directionality, meaning it has a defined "start" and an "end." This intrinsic polarity is fundamental to how proteins are synthesized, fold, and function.
Peptide sequences are always read from left to right, starting from the N-terminus and proceeding sequentially towards the C-terminus.
Each amino acid unit within the peptide chain, after forming peptide bonds, is referred to as an amino acid residue. This term emphasizes that each amino acid has lost the elements of water (a hydrogen atom from its amino group and a hydroxyl group from its carboxyl group) when participating in the formation of a peptide bond. Within the chain, only the R-group and the α-carbon, along with parts of the backbone, remain.
To simplify the writing and reading of often very long protein sequences, standard abbreviations are universally used for the 20 common genetically encoded amino acids:
| Amino Acid | Three-Letter Code | One-Letter Code |
|---|---|---|
| Alanine | Ala | A |
| Arginine | Arg | R |
| Asparagine | Asn | N |
| Aspartate | Asp | D |
| Cysteine | Cys | C |
| Glutamine | Gln | Q |
| Glutamate | Glu | E |
| Glycine | Gly | G |
| Histidine | His | H |
| Isoleucine | Ile | I |
| Leucine | Leu | L |
| Lysine | Lys | K |
| Methionine | Met | M |
| Phenylalanine | Phe | F |
| Proline | Pro | P |
| Serine | Ser | S |
| Threonine | Thr | T |
| Tryptophan | Trp | W |
| Tyrosine | Tyr | Y |
| Valine | Val | V |
Note: For cases where the exact amide status is unknown or ambiguous:
When asked to "name" a peptide or write its sequence, you list the amino acid residues in order from the N-terminus to the C-terminus, using their standard abbreviations.
Let's carefully examine this peptide structure to determine its sequence.
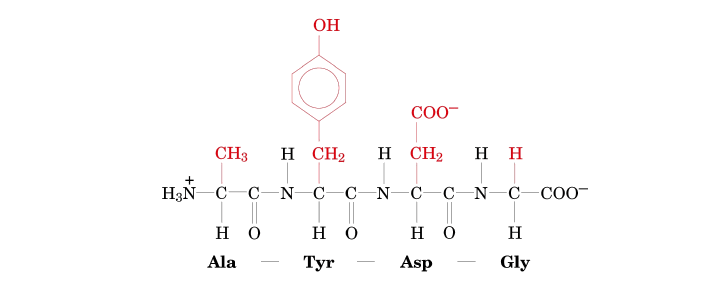
Step-by-step identification:
Beyond the R-group classification (which is by far the most common in structural biochemistry and determines an amino acid's direct contribution to protein structure and interaction), amino acids can also be classified based on their chemical properties, nutritional requirements, and metabolic fates. These classifications provide different lenses through which to understand their roles in biology.
This classification often overlaps with the R-group classification (e.g., polar, nonpolar, charged) but can highlight specific chemical properties not solely related to polarity or charge. It categorizes amino acids based on the overall nature of their side chains and their behavior in solution.
This classification is from a dietary perspective, particularly for humans. It categorizes amino acids based on whether the human body can synthesize them de novo (from scratch) or if they must be obtained through the diet.
The body cannot synthesize these, so they must be obtained from the diet. There are 9: Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Threonine, Tryptophan, and Valine.
The body can synthesize these from other compounds, so they are not required in the diet. Examples include Alanine, Aspartate, Glycine, and Serine.
Normally non-essential, but become essential during illness, rapid growth, or stress. Examples include Arginine, Cysteine, Tyrosine, and Glutamine.
Mnemonic (a common one, often extended): PVT TIM HALL (Phenylalanine, Valine, Threonine, Tryptophan, Isoleucine, Methionine, Histidine, Arginine, Leucine, Lysine). Note: Arginine is often considered conditionally essential, see below.
This classification categorizes amino acids based on the fate of their carbon skeletons after the amino group has been removed (a process called deamination or transamination). This dictates how the body uses them for energy production or to synthesize other crucial biomolecules.
These can be converted into glucose via gluconeogenesis. Their carbon skeletons are degraded to intermediates like pyruvate or oxaloacetate. Examples include Alanine, Glycine, and Serine.
These can be converted into ketone bodies or their precursors (acetyl-CoA). Leucine and Lysine are the only two amino acids that are purely ketogenic.
These can be degraded into intermediates that form both glucose and ketone bodies. Examples include Isoleucine, Phenylalanine, Tyrosine, and Tryptophan.
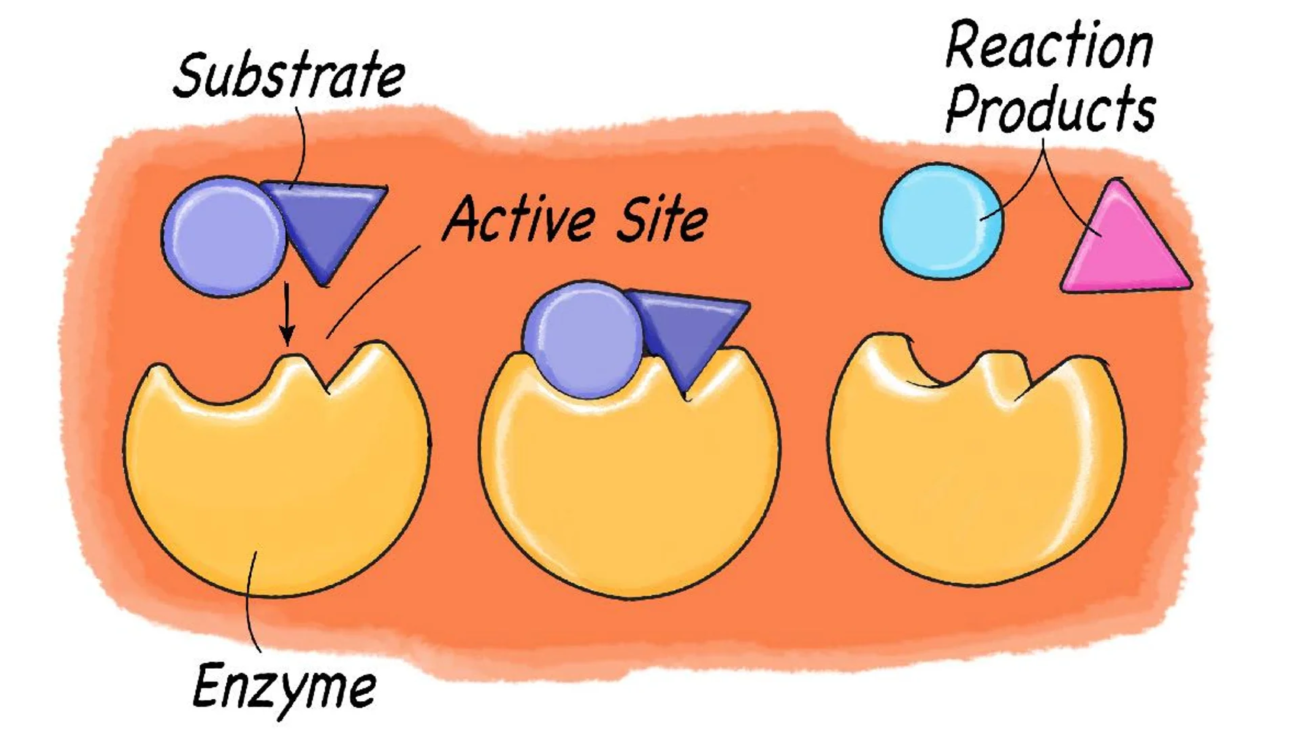
The Biuret test is a classic qualitative (and semi-quantitative) chemical test used to detect the presence of proteins and peptides in a solution.
The Biuret test specifically detects the presence of peptide bonds. It relies on the ability of copper(II) ions (Cu2+) in an alkaline solution to form a distinctive violet-colored chelate complex with compounds containing two or more peptide bonds. A single amino acid or a dipeptide will not give a positive Biuret test.
Proteins are not just linear chains of amino acids; they fold into precise, intricate three-dimensional structures that are absolutely essential for their biological function. This complex folding process can be described at four hierarchical levels.
Key Types: The two most common, stable, and well-defined types of secondary structure are:
(A classification based on overall 3D shape, largely determined by tertiary structure)
The biological function of a protein is precisely linked to its precise three-dimensional structure. The journey from a linear polypeptide chain to a biologically active, folded protein is a complex and highly regulated process known as protein folding. Conversely, the loss of this critical 3D structure, leading to loss of function, is termed denaturation.
Definition: Protein folding is the spontaneous (or chaperon-assisted) process by which a newly synthesized or unfolded polypeptide chain acquires its intricate, specific, and functionally active three-dimensional conformation (its native state). This precise 3D structure is determined primarily by its primary amino acid sequence.
The folding of a protein from a vast number of possible conformations to a single, stable native state is often referred to as the "folding problem." This process is generally understood in terms of an energy funnel or energy landscape:
The acquisition and maintenance of the native 3D structure are driven and stabilized by a combination of weak non-covalent interactions and, occasionally, strong covalent bonds. These interactions occur between amino acid R-groups and between backbone atoms:
Definition: Molecular chaperones are a diverse and essential group of proteins that assist in the proper folding of other proteins. They do not become part of the final functional protein themselves; rather, they act as "helpers" or "escorts" in the folding process. They are particularly crucial under cellular stress conditions (like heat shock) or for newly synthesized proteins, guiding them through potentially hazardous folding pathways.
Major Chaperone Families: Examples include the Hsp70 family (which binds to nascent chains), Hsp90 (involved in the maturation of signaling proteins), and chaperonins like GroEL/GroES (which provide an "isolation chamber" for protein folding).
Definition: Denaturation is the process by which a protein loses its specific, biologically active, native three-dimensional conformation. This loss of structure typically results in a loss of biological function.
Various physical and chemical agents can cause denaturation by interfering with the weak forces that maintain protein structure:
Mechanism: Increases kinetic energy, causing vibrations that disrupt weak non-covalent interactions like hydrogen bonds and hydrophobic interactions. Effect: Causes unfolding, often irreversibly, like cooking an egg.
Mechanism: Alters the ionization state of acidic and basic R-groups, disrupting crucial ionic bonds (salt bridges) and hydrogen bonding patterns. Effect: Causes charge repulsion and destabilizes the native conformation.
Mechanism: Less polar than water, these solvents (e.g., ethanol, acetone) disrupt and dissolve the internal hydrophobic core of proteins. Effect: Weakens the hydrophobic effect, leading to unfolding and precipitation.
Mechanism: Amphipathic molecules (e.g., SDS) bind to and disrupt hydrophobic regions, coating the protein with charge. Effect: Leads to complete unfolding into a random coil, useful in laboratory techniques.
Mechanism: Ions like Pb²⁺ or Hg²⁺ react strongly with sulfhydryl (-SH) groups and charged R-groups. Effect: Disrupts disulfide and ionic bonds, often causing irreversible denaturation and enzyme inactivation.
Mechanism: Small molecules (e.g., urea, guanidinium chloride) disrupt the structure of water and form H-bonds with the protein. Effect: Weakens the hydrophobic effect and disrupts internal H-bonds, causing complete unfolding.
Mechanism: Vigorous shaking, grinding, or shearing applies physical force that can break weak non-covalent interactions. Effect: Causes unfolding and aggregation as exposed hydrophobic regions interact, such as when whipping egg whites.
Despite the cellular machinery dedicated to ensuring proper protein folding, including a battery of molecular chaperones, errors can (and do) occur.
Proteins may fail to achieve their correct native state, or they may denature and subsequently refold improperly.
The accumulation of these misfolded proteins can have profound and often devastating consequences, leading to a wide array of severe diseases, prominently featuring neurodegenerative disorders. These conditions underscore the critical link between protein structure, function, and cellular health.
While the specific proteins and affected tissues vary, a common set of pathological mechanisms underlies most protein misfolding diseases:
A Classic Example of a Point Mutation Leading to Aberrant Assembly
Misfolded/Mutated Protein: Hemoglobin (Hb). Specifically, a single-point mutation converts normal hemoglobin (HbA) to sickle hemoglobin (HbS) by replacing a polar glutamate with a nonpolar valine.
Mechanism: This substitution creates a "sticky" hydrophobic patch on the surface of deoxy-HbS. Under low oxygen conditions, these patches cause HbS molecules to polymerize into long, rigid, insoluble fibers that distort red blood cells into a rigid sickle shape.
Effect: The sickled cells are fragile (causing anemia) and rigid, leading to blockage of small blood vessels (vaso-occlusive crises), intense pain, and organ damage. It is a prime example of how a single amino acid change can have catastrophic physiological consequences.
A Dual-Protein Pathology
Misfolded Proteins: Primarily involves two proteins: Beta-amyloid (Aβ) and Tau.
Mechanism: Aβ is a peptide that misfolds and aggregates extracellularly to form insoluble amyloid plaques between neurons. The Tau protein becomes hyperphosphorylated, detaches from microtubules, and aggregates intracellularly to form neurofibrillary tangles (NFTs) inside neurons.
Effect: The accumulation of both plaques and tangles is thought to cause widespread neuronal dysfunction and death, leading to progressive cognitive decline, severe memory loss, and dementia.
Synucleinopathy
Misfolded Protein: Alpha-synuclein, a protein involved in synaptic vesicle regulation.
Mechanism: Alpha-synuclein misfolds and aggregates into intracellular inclusions called Lewy bodies and Lewy neurites. These aggregates primarily affect dopaminergic neurons in the substantia nigra region of the brain.
Effect: The progressive loss of these dopamine-producing neurons leads to a severe dopamine deficiency, causing the characteristic motor symptoms of Parkinson's, including tremor, rigidity, slowness of movement (bradykinesia), and postural instability.
Transmissible Spongiform Encephalopathies
Misfolded Protein: Prion protein (PrP). This disease is unique because the misfolded protein itself is infectious.
Mechanism: A normal cellular protein (PrPC) misfolds into an abnormal, protease-resistant isoform (PrPSc). This infectious PrPSc then acts as a template, forcing other normal PrPC molecules to adopt the misfolded conformation in a self-propagating chain reaction.
Effect: The accumulation of PrPSc aggregates causes widespread neuronal death and a "spongiform" (vacuolated) appearance in the brain, leading to rapidly progressive and fatal neurodegeneration. Examples include Creutzfeldt-Jakob Disease (CJD) in humans and "Mad Cow Disease" (BSE) in cattle.
A Quality Control Error
Misfolded Protein: Cystic Fibrosis Transmembrane Conductance Regulator (CFTR), a chloride ion channel.
Mechanism: A common mutation (ΔF508) causes the CFTR protein to misfold. While it might still be partially functional, the cell's own quality control machinery in the endoplasmic reticulum recognizes the misfolded protein and targets it for premature degradation before it can reach the cell membrane.
Effect: The lack of functional CFTR channels at the cell surface impairs chloride ion transport, leading to thick, sticky mucus in the lungs, pancreas, and other organs, causing chronic infections, respiratory failure, and malabsorption.
A 2-year-old boy from Mukono district is admitted to the hospital presenting with a constellation of acute symptoms: recurrent, excruciating severe bone pain affecting his hands, feet, and sternum for the past 3 days, accompanied by noticeable jaundice and profound fatigue. His parents report previous, similar episodes.
Laboratory findings on admission reveal:
Based on these findings, a diagnosis of Vaso-occlusive crisis and severe anemia due to Sickle Cell Disease was made.
Detailed Explanation of the Amino Acid Change:
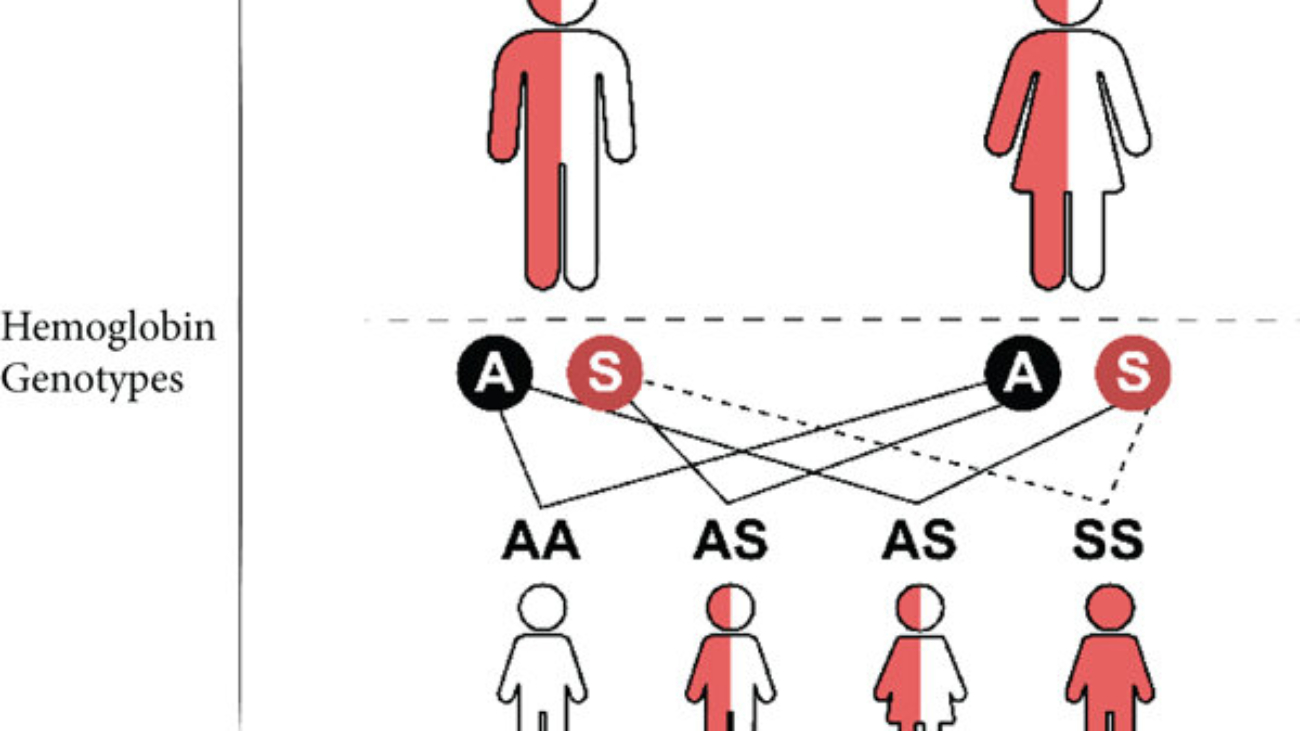
The genetic basis of Sickle Cell Disease (SCD) in this patient, as confirmed by the presence of HbS, lies in a single-point mutation within the gene encoding the beta-globin chain of hemoglobin. This seemingly minor alteration in the DNA sequence triggers a profound change at the protein level:
Molecular Mechanism and Clinical Manifestations:
The single amino acid substitution of Valine for Glutamate at position 6 of the beta-globin chain profoundly alters the molecular behavior of hemoglobin S (HbS), particularly under conditions of low oxygen. This chain of events directly explains the patient's clinical presentation:
Role of Amino Acid Chemistry in Therapeutic Approaches to SCD:
Understanding the precise amino acid change and its chemical consequences is fundamental to designing and developing targeted therapies for SCD. Many current and emerging treatments aim to counteract the effects of the Valine substitution by modulating protein-protein interactions, altering the oxygen affinity of HbS, or promoting the production of alternative hemoglobin forms.
In summary, a deep understanding of the chemical properties of amino acids and how their interactions govern protein structure and function is paramount. Therapies for SCD leverage this knowledge to develop molecules that either directly prevent the abnormal hydrophobic interactions (like Voxelotor), indirectly modify the cellular environment to reduce sickling (like Hydroxyurea), or, in the future, correct the genetic error at its source.
Mrs. Eleanor Vance, an 82-year-old retired schoolteacher, is brought to the neurology clinic by her worried daughter. Over the past 5 years, Mrs. Vance has exhibited a gradual and progressive decline in her cognitive abilities. Initially, it was subtle memory lapses, such as forgetting names or misplacing keys. More recently, she has struggled with complex tasks like managing her finances, preparing meals, and following conversations. Her daughter reports that Mrs. Vance frequently repeats herself, gets disoriented in familiar surroundings, and occasionally exhibits mood swings and agitation. There is no history of stroke or significant head trauma. A physical and neurological examination reveals no focal deficits, but a mini-mental state examination (MMSE) score indicates significant cognitive impairment. Brain imaging (MRI) shows generalized cerebral atrophy, particularly pronounced in the hippocampus and cerebral cortex, but no evidence of tumors or vascular lesions.
Based on the clinical presentation and diagnostic findings, a presumptive diagnosis of Alzheimer's Disease is made.
Primary Protein and Origin:
The primary protein involved in the formation of amyloid plaques in Alzheimer's Disease is beta-amyloid (Aβ) peptide.
Aβ is not synthesized as a standalone protein but is a small fragment (typically 38-43 amino acids long) derived from a much larger, integral transmembrane protein called the Amyloid Precursor Protein (APP). The production of Aβ occurs through the sequential proteolytic cleavage of APP by two different enzymes: β-secretase and γ-secretase. The longer form, Aβ42, is particularly prone to aggregation and is considered the more pathogenic species.
Misfolding and Aggregation and Contribution to Pathology:
Normally, Aβ peptides exist as soluble monomers. However, in AD, Aβ undergoes a critical misfolding event:
Primary Protein Involved:
The primary protein involved in the formation of neurofibrillary tangles (NFTs) is Tau protein.
Tau is a microtubule-associated protein (MAP) that is highly abundant in neurons. Its primary physiological function is to stabilize microtubules, which are essential components of the neuronal cytoskeleton for maintaining structure and facilitating intracellular transport.
Post-Translational Modification Initiating Misfolding:
The specific post-translational modification that initiates the misfolding and subsequent aggregation of tau protein in AD is hyperphosphorylation.
In AD, tau becomes abnormally and excessively phosphorylated at multiple sites. This hyperphosphorylation causes it to detach from microtubules and undergo a conformational change, exposing regions that facilitate self-association. It then misfolds and aggregates into insoluble helical filaments, eventually forming large NFTs inside neurons.
How Aggregation Leads to Neuronal Dysfunction:
The accumulation of NFTs within neurons leads to profound neuronal dysfunction and ultimately cell death by:
Aggregated proteins in Alzheimer's Disease are particularly problematic in post-mitotic cells like neurons due to a confluence of factors related to their unique cellular biology and the limitations of their protein quality control systems.
Test your knowledge with these 40 questions.
Question 1/40
Here are your results, .
Your Score
38/40
95%
At their most fundamental level, carbohydrates are organic molecules composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The most common and simplified general formula you'll see for carbohydrates is (CH₂O)n, where 'n' represents the number of carbon atoms, and 'n' is 3 or greater.
However, a more chemically precise definition,
Carbohydrates are polyhydroxy aldehydes or polyhydroxy ketones, or substances that yield these compounds upon hydrolysis.
Polyhydroxy: This is a critical term. "Poly-" means many, and "hydroxy" refers to the hydroxyl group (-OH). So, a polyhydroxy compound is one that contains multiple hydroxyl (-OH) groups attached to different carbon atoms. These hydroxyl groups are responsible for many of the characteristic properties of carbohydrates, such as their solubility in water and their ability to form hydrogen bonds.
Aldehyde: An aldehyde is an organic functional group characterized by a carbonyl group (C=O) where the carbon atom is bonded to at least one hydrogen atom and one other carbon atom (or a second hydrogen atom). It resides at the end of a carbon chain. Visualizing it: R-CHO where R is the rest of the carbon chain.
Ketone: A ketone is another organic functional group, also characterized by a carbonyl group (C=O), but in a ketone, the carbon atom of the carbonyl group is bonded to two other carbon atoms. It resides within a carbon chain, not at the end. Visualizing it: R-CO-R' where R and R' are the rest of the carbon chains.
Substances that yield these compounds upon hydrolysis: This part of the definition accounts for more complex carbohydrates (like disaccharides and polysaccharides). These larger molecules don't directly fit the polyhydroxy aldehyde/ketone description, but when they are broken down (hydrolyzed) by adding water, they release smaller units that do fit the description (monosaccharides).
In simpler terms: Carbohydrates are organic molecules that have several alcohol-like (-OH) groups and, in their simplest form, also contain either an aldehyde group or a ketone group.
Photosynthesis is the process where plants use sunlight, water, and carbon dioxide to make glucose and oxygen.
Is a biological process carried out by plants, algae, and some types of bacteria.
For plants: Glucose is their immediate energy source, and starch is how they store that energy. Cellulose forms their cell walls, giving them structure.
For animals (and humans): We are heterotrophs (meaning "other-feeders"). Because plants are autotrophs, (food makers). We cannot perform photosynthesis. We obtain our carbohydrates (and energy) by eating plants directly (e.g., fruits, vegetables, grains) or by eating animals that have eaten plants. When we consume these plant-derived carbohydrates, our digestive system breaks them down into simpler sugars (like glucose), which our cells then use for energy.
Primary Energy Source for Living Organisms: Carbohydrates, particularly glucose, serve as the most immediate and readily available fuel source for nearly all living cells. Through cellular respiration, glucose is metabolized to produce ATP (adenosine triphosphate), that powers vital cellular processes such as muscle contraction, nerve impulse transmission, and active transport.
Storage Form of Energy: Allowing organisms to maintain energy reserves for periods of high demand or scarcity.
Structural Components: Carbohydrates provide structural integrity and protection to cells and tissues across diverse life forms.
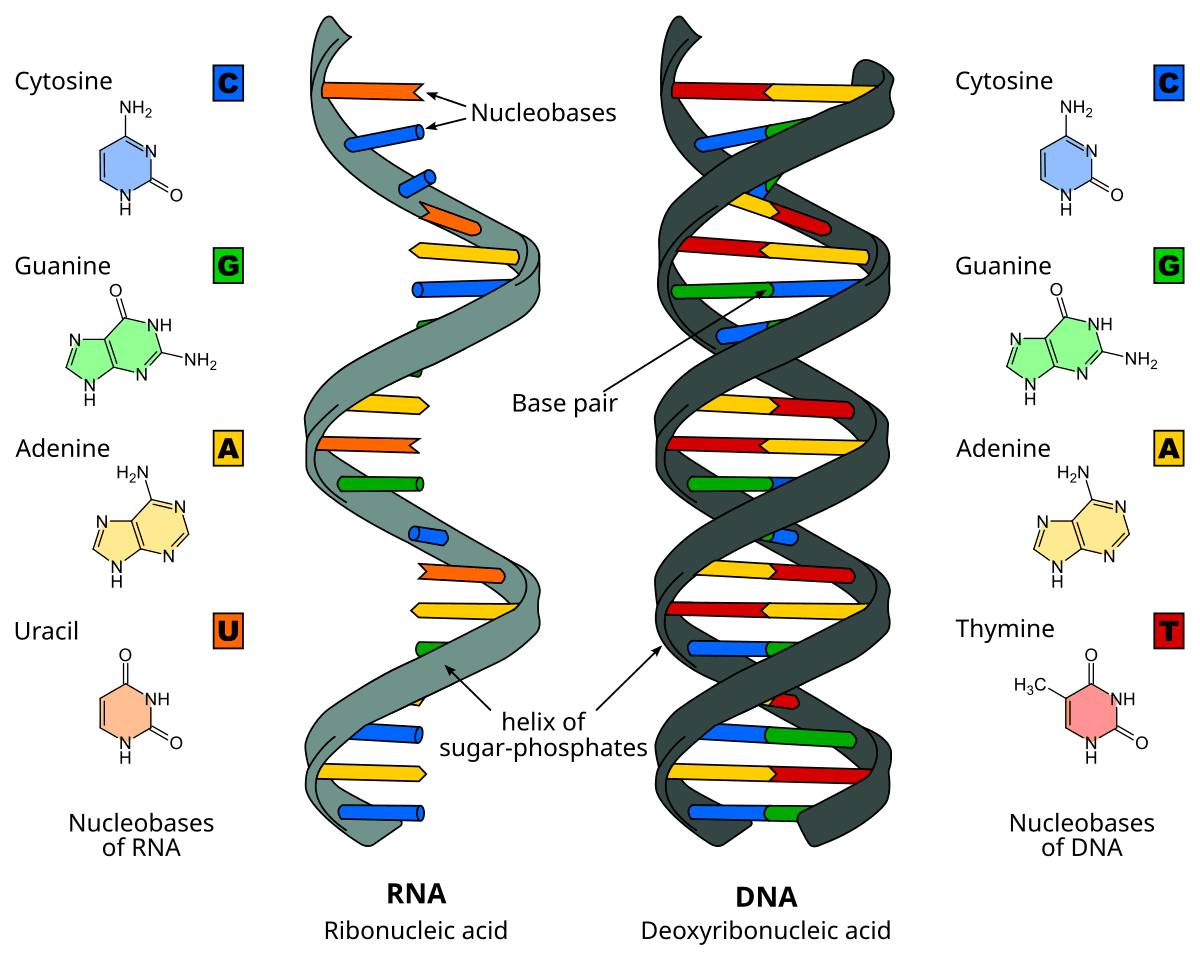
Constituent of Nucleic Acids: Specific five-carbon sugars are integral to the backbone of the genetic material of all life.
Dietary Fibre (Non-digestible Carbohydrates): Like cellulose, hemicellulose, and pectin, are not digestible by human enzymes but are essential for digestive health. Termed dietary fibre, they provide bulk to stool, aid in regular bowel movements, prevent constipation, and can contribute to gut microbiome health.
Lubrication, Cellular Intercommunication, & Immune Response: Glycoproteins and glycolipids on cell surfaces, are for cellular processes:
Detoxification Role (e.g., Glucuronic Acid): Glucuronic acid, a derivative of glucose, is vital in the liver. It conjugates (attaches) to various toxic substances, drugs, and metabolic waste products, making them more water-soluble and easier for the body to excrete through urine or bile. This process is essential for clearing harmful compounds from the system.
As mentioned, the empirical formula for many simple carbohydrates is (CH₂O)n. For example:
NB: not all carbohydrates strictly adhere to this exact ratio (e.g., deoxyribose, which has one less oxygen atom than expected, or some modified carbohydrates).
Carbohydrates are classified into groups based on the number of their constituent sugar units. The term "saccharide" (from the Greek "sakcharon" meaning sugar) is often used interchangeably with carbohydrate.
There are three primary classes of carbohydrates:
Further Classification: Monosaccharides can be further categorized by:
Commonest Type: Disaccharides: The most prevalent type of oligosaccharide consists of two monosaccharide units joined together. Examples of Disaccharides:
Formation: Disaccharides are formed by a dehydration (condensation) reaction where a water molecule is removed as two monosaccharides form a glycosidic bond.
Other Oligosaccharides (3-10 units): Examples: Raffinose (3 units - Gal-Glu-Fru), Stachyose (4 units - Gal-Gal-Glu-Fru). These are often found in legumes and can contribute to flatulence due to their non-digestibility by human enzymes until they reach gut bacteria.
Properties: Due to their large size, polysaccharides have a high molecular weight, are generally not sweet, and can be insoluble or form colloidal dispersions in water. Examples: Polysaccharides are diverse and can be broadly categorized by their primary biological function:
Monosaccharides, also known as "simple sugars," are the most basic units of carbohydrates. They are single sugar molecules that cannot be hydrolyzed (broken down by water) into simpler carbohydrate units.
They serve as the primary fuel source for cells, the fundamental building blocks for more complex carbohydrates (disaccharides, oligosaccharides, and polysaccharides), and as crucial components in nucleic acids (DNA, RNA) and other vital biomolecules.
(CH2O)n, where 'n' usually ranges from 3 to 7, though some rarer forms can have up to 9 carbon atoms. This formula highlights that for every carbon atom, there is approximately one water molecule equivalent, hence "carbo-hydrate."
Every monosaccharide possesses defining chemical characteristics that dictate its reactivity and biological role:
One Carbonyl Group (C=O): This is the most reactive functional group and determines whether the sugar is an aldose or a ketose.
Aldehyde Group (R-CHO): If the carbonyl group is located at the end of the carbon chain (C1), it forms an aldose. Aldehyde groups are readily oxidized, making aldoses reducing sugars.Ketone Group (R-CO-R'): If the carbonyl group is located at any position other than the end of the carbon chain (typically C2 in the physiologically important ketoses), it forms a ketose. Ketones are generally less reactive than aldehydes, but ketoses can isomerize to aldoses, allowing them to also act as reducing sugars under certain conditions.Multiple Hydroxyl Groups (-OH): At least one hydroxyl group is present on every carbon atom that doesn't bear the carbonyl group.
Polarity and Solubility: The presence of numerous highly polar hydroxyl groups makes monosaccharides exceptionally hydrophilic (water-loving) and therefore highly soluble in water. This is crucial for their transport in aqueous biological environments (e.g., blood plasma, cytoplasm).Reactivity: These hydroxyl groups are also reactive, participating in various biochemical reactions, including:
Monosaccharides are systematically classified based on two primary structural features:
1. The Nature of the Carbonyl Group:
2. The Number of Carbon Atoms in the Chain:
Examples: Glyceraldehyde (an aldotriose, important in glycolysis) and Dihydroxyacetone (a ketotriose, also in glycolysis).Examples: Erythrose (an aldotetrose, involved in pentose phosphate pathway).Examples:Examples:Examples: Sedoheptulose (a ketoheptose, an intermediate in the pentose phosphate pathway).By combining these two classification methods, we can precisely describe any monosaccharide:
This is a profoundly important characteristic of monosaccharides, especially for biological recognition.
In aqueous solutions (like within the body), monosaccharides with 5 or more carbons (and even some 4-carbon sugars) spontaneously cyclize (form rings) rather than existing as open chains. This is a crucial aspect of their structure and reactivity.
Beyond the basic forms, monosaccharides can be modified for specialized roles:
As established, isomers are molecules that possess the same molecular formula (meaning they have identical numbers and types of atoms) but exhibit a different arrangement of those atoms. This difference in arrangement leads to distinct chemical and/or physical properties. The existence of isomers is foundational to the vast diversity of organic molecules, particularly carbohydrates, where subtle structural differences dictate profound biological outcomes.
We categorize isomers into two primary types: Structural (Constitutional) Isomers and Stereoisomers.
Structural isomers are characterized by having the same molecular formula but a different connectivity or sequence of bonded atoms. This means the atoms are connected to each other in a fundamentally different order, resulting in different parent structures. While less common among monosaccharides themselves (due to the strict (CH2O)n formula and functional group placement rules), understanding them provides a crucial foundation.
There are three main sub-types of structural isomerism:
Definition: These isomers differ in the arrangement of the carbon skeleton itself. The carbon atoms can be arranged in a straight chain, a branched chain, or a ring.
Example (General Chemistry): For the molecular formula C4H10 (Butane):
CH3
|
CH3 - CH - CH3
Both have four carbons and ten hydrogens, but their carbon backbones are arranged differently.
Relevance to Monosaccharides: Not typically observed within the monosaccharide family (e.g., you won't find a branched-chain glucose isomer that is still a 6-carbon monosaccharide), but important for understanding overall carbohydrate structure (e.g., branched vs. unbranched polysaccharides).
Definition: These isomers have the same carbon skeleton and the same functional groups, but the functional group(s) or substituent(s) are located at different positions on the carbon chain.
Example 1 (General Chemistry): Butan-1-ol vs. Butan-2-ol (Molecular formula C4H10O)
OH
|
CH3 - CH - CH2 - CH3
Relevance to Monosaccharides: While the carbonyl group defines the aldose/ketose classification, the positions of hydroxyl groups define different sugars once cyclized (e.g., the position of the anomeric -OH for α/β anomers, though this is more accurately a stereoisomer difference). Phosphorylated sugars (e.g., glucose-6-phosphate vs. glucose-1-phosphate) could be considered positional isomers if viewing the phosphate as a "substituent" on the base sugar.
Definition: These isomers have the same molecular formula but possess different functional groups. This means the atoms are connected in such a way that they form entirely different classes of compounds with distinct chemical properties.
Example 1 (General Chemistry): Ethanol vs. Dimethyl Ether (Molecular formula C2H6O)
These are vastly different compounds: ethanol is a liquid at room temperature, while dimethyl ether is a gas.
Example 2 (Directly Applicable to Monosaccharides): Glucose vs. Fructose (Molecular formula C6H12O6)
Biological/Clinical Significance: This is a critical distinction! While both are hexoses and primary energy sources, their initial metabolic pathways differ. Glucose enters glycolysis directly; fructose must first be converted into glycolytic intermediates, primarily in the liver. Defects in fructose metabolism (e.g., hereditary fructose intolerance) can lead to severe health issues.
Stereoisomers have the same molecular formula and the same connectivity (bonding sequence) of atoms, but they differ only in the 3D arrangement of their atoms in space. This spatial arrangement, or configuration, is paramount in biology because enzymes and receptors are exquisitely sensitive to the precise three-dimensional shape of molecules.
There are two major types of stereoisomerism: Geometrical Isomerism and Optical Isomerism.
Definition: This type of isomerism arises when there is restricted rotation around a bond, most commonly a carbon-carbon double bond (C=C), or within a ring structure. The different groups attached to the carbons involved in the restricted bond can be on the same side (cis) or opposite sides (trans) of that bond.
Requirement: Each carbon in the double bond (or in the ring that restricts rotation) must be attached to two different groups.
Biological Significance: While not directly applicable to simple monosaccharides (which don't have C=C double bonds in their carbon backbone), this type of isomerism is vital in other biological molecules like:
Example (General Chemistry): 2-Butene (Molecular formula C4H8)
CH3 CH3
\ /
C = C
/ \
H H
CH3 H
\ /
C = C
/ \
H CH3
These are not interconvertible without breaking the double bond.
Optical isomerism refers to compounds that differ in their ability to rotate plane-polarized light. This property arises from the presence of chiral centers within the molecule.
Definition: Stereoisomers that are non-superimposable mirror images of each other. They contain at least one chiral center.
Properties:
D- and L- Designation: In biochemistry, the D- and L- system is universally used, especially for carbohydrates and amino acids. It relates to the configuration of the chiral carbon furthest from the primary functional group (carbonyl in sugars).
Example (Monosaccharide): D-Glyceraldehyde vs. L-Glyceraldehyde (C3H6O3)
Glyceraldehyde has one chiral center (C2).
CHO CHO
| |
H-C-OH HO-C-H <-- Chiral carbon
| |
CH2OH CH2OH
D-Glyceraldehyde L-Glyceraldehyde
These are exact mirror images and cannot be superimposed.
Biological/Clinical Significance: Enzymes are typically specific for one enantiomeric form. For example, our digestive enzymes can break down D-glucose but not L-glucose. If we consumed L-glucose, it would pass through our digestive system largely undigested and unabsorbed, providing no caloric value. This specificity is why synthetic drugs often need to be produced as a single enantiomer to ensure efficacy and avoid side effects.
Definition: Stereoisomers that are not mirror images of each other. They arise in molecules with two or more chiral centers.
Properties:
Biological/Clinical Significance: The subtle differences in 3D structure between diastereomers allow for distinct recognition by biological systems. Our bodies distinguish between glucose, galactose, and mannose, even though they are all hexoses with the same functional group and formula.
Sub-types of Diastereomers (Crucial for Monosaccharides):
Epimers:
CHO CHO
| |
H-C-OH H-C-OH
| |
HO-C-H HO-C-H
| |
H-C-OH HO-C-H <-- *Difference at C4*
| |
H-C-OH H-C-OH
| |
CH2OH CH2OH
D-Glucose D-Galactose
Anomers:
CH2OH CH2OH
/ /
O O
/ \ / \
C---C OH HO-C---C
| | | |
C-----C C-----C
\ / \ /
OH OH OH OH
α-D-Glucopyranose β-D-Glucopyranose
(OH on C1 is 'down') (OH on C1 is 'up')
| Isomer Type | Definition | Same Formula? | Same Connectivity? | Different 3D? | Biological Relevance/Examples |
|---|---|---|---|---|---|
| 1. Structural Isomers | |||||
| a. Chain | Different carbon skeleton arrangement | Yes | No | Yes | Less common for monosaccharides; relevant for overall polysaccharide branching. |
| b. Positional | Same skeleton & functional group, but group position differs | Yes | No | Yes | E.g., Glucose-1-phosphate vs. Glucose-6-phosphate (metabolic intermediates). |
| c. Functional | Same formula, but atoms arranged to form different functional groups | Yes | No | Yes | Glucose (aldose) vs. Fructose (ketose) – same molecular formula (C6H12O6), but different metabolic pathways, significant in diabetes and fructose intolerance. |
| 2. Stereoisomers | |||||
| a. Geometrical | Different spatial arrangement around a restricted bond (e.g., C=C, ring) | Yes | Yes | Yes | Not common in simple monosaccharides, but vital in fatty acids (cis/trans fats) and vision pigments (retinal cis/trans isomerization). |
| b. Optical | Differ in ability to rotate plane-polarized light (due to chiral centers) | Yes | Yes | Yes | Defines how molecules interact with living systems. |
| i. Enantiomers | Non-superimposable mirror images | Yes | Yes | Yes | D-sugars vs. L-sugars: Mammalian enzymes almost exclusively recognize D-sugars (e.g., D-glucose). L-sugars are often metabolically inert. Crucial for drug chirality and efficacy. |
| ii. Diastereomers | Stereoisomers that are NOT mirror images (multiple chiral centers) | Yes | Yes | Yes | Allow for distinct recognition by enzymes. |
| • Epimers | Diastereomers differing at ONLY ONE chiral center | Yes | Yes | Yes | D-Glucose vs. D-Galactose (C4 epimers), D-Glucose vs. D-Mannose (C2 epimers). Enzymatic epimerization is important for interconverting sugars in metabolism (e.g., galactose to glucose). Metabolic disorders like galactosemia stem from this. |
| • Anomers | Diastereomers formed during ring closure, differing at the anomeric carbon (C1 for aldose, C2 for ketose) | Yes | Yes | Yes | α-D-Glucose vs. β-D-Glucose. Determines the type of glycosidic bond in polysaccharides: starch (α-linkages, digestible) vs. cellulose (β-linkages, indigestible). Impacts carbohydrate digestion and fiber function. |
The most common and biologically significant monosaccharides are the hexoses, meaning they are sugars with six carbon atoms (C6H12O6). Among these, three stand out: glucose, fructose, and galactose. Their structural differences, though subtle, dictate distinct metabolic fates and clinical implications.
Classification: Aldohexose (an aldehyde sugar with six carbons). Specifically, α-D-glucopyranose and β-D-glucopyranose are the most prevalent cyclic forms in solution.
Common Name: Often referred to as "grape sugar," "dextrose" (due to its dextrorotatory property, rotating plane-polarized light to the right), or most commonly, "blood sugar."
Biological Importance: The undisputed king of sugars in human metabolism.
Clinical Significance:
Classification: Also an aldohexose. Specifically, a C4 epimer of D-glucose.
Common Name: Sometimes called "milk sugar" because it's a component of lactose (milk sugar).
Biological Importance:
Clinical Significance:
Classification: Ketohexose (a ketone sugar with six carbons). Primarily exists in its five-membered ring form, β-D-fructofuranose, when free in solution or in disaccharides.
Common Name: Known as "fruit sugar" or "levulose" (due to its strong levorotatory property, rotating plane-polarized light to the left) because it's abundant in fruits, honey, and some vegetables.
Biological Importance:
Clinical Significance:
This is a very important chemical property of monosaccharides, particularly relevant in diagnostic tests (like for diabetes) and in food chemistry.
A reducing sugar is any sugar that is capable of acting as a reducing agent. This means it can donate electrons to another molecule, thereby reducing that molecule (and itself becoming oxidized).
In the context of sugars, this reducing power comes from the presence of a free aldehyde (-CHO) group or a free ketone (C=O) group that can isomerize to an aldehyde group under certain conditions (e.g., in alkaline solutions).
Aldehyde Oxidation: The aldehyde group is easily oxidized to a carboxylic acid, releasing electrons in the process:
R-CHO (Aldehyde) + Oxidizing Agent --> R-COOH (Carboxylic Acid) + Reduced Agent
These tests rely on the ability of the aldehyde group (or isomerized ketone) to reduce a metal ion, often resulting in a color change or precipitate.
Benedict's Test:
Fehling's Test: Similar to Benedict's test, uses Cu2+ ions in an alkaline solution (with tartrate as a chelator) to detect reducing sugars.
Diabetes Diagnosis and Monitoring (Historical & Current):
Food Chemistry:
Renal Physiology: The renal threshold for glucose (typically 180 mg/dL or 10 mmol/L) is the plasma glucose concentration above which glucose starts to appear in the urine because the renal tubules' capacity to reabsorb glucose is saturated. Understanding the reducing nature of glucose helps explain why its presence in urine indicates a physiological abnormality.
Having understood monosaccharides as individual sugar units, we now see how these units combine to form larger, molecules. This combination is facilitated by a special type of covalent bond known as the glycosidic bond.
A glycosidic bond is a covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
When two monosaccharides link together, this bond is specifically referred to as an O-glycosidic bond because it involves an oxygen atom.
The bond forms when one monosaccharide's anomeric hydroxyl group reacts with another's hydroxyl group, releasing a water molecule (a condensation reaction).
The orientation of the anomeric hydroxyl group (which dictates the configuration of the anomeric carbon) is critical in determining the type of glycosidic bond formed:
To precisely describe a glycosidic bond, we specify which carbon atoms of each monosaccharide are linked. For example:
Disaccharides are carbohydrates formed when two monosaccharide units are joined together by a single glycosidic bond.
Three most common disaccharides:
The anomeric carbon is indeed the special carbon in a cyclic sugar that was once the aldehyde or ketone carbon in the open chain. It's unique because:
Polysaccharides are long chains of monosaccharide units (ranging from hundreds to many thousands) linked together by glycosidic bonds. They are polymers, serving diverse and essential biological functions such as energy storage, structural support, cell-cell communication, and lubrication. Their complex structures arise from the type of monosaccharides, the length of the chains, the types of glycosidic bonds (α or β), and the presence of branching.
These are polysaccharides composed of only one type of monosaccharide unit. The most common building block is glucose, but other monosaccharides can also form homopolysaccharides.
These are polysaccharides composed of two or more different types of monosaccharide units. They are often more complex and include substances like glycosaminoglycans (GAGs), which are important components of connective tissues, lubricants, and the extracellular matrix (ECM).
What they are: Long, unbranched polysaccharide chains made of repeating disaccharide units. Each disaccharide unit typically consists of an amino sugar (like N-acetylglucosamine or N-acetylgalactosamine) and an uronic acid (like D-glucuronic acid or L-iduronic acid). They are highly negatively charged due to the presence of sulfate groups (e.g., chondroitin sulfate, keratan sulfate, heparan sulfate, dermatan sulfate) and carboxyl groups on the uronic acids.
Key Characteristics & Functions:
Important Examples & Their Locations/Functions:
What they are: These are special macromolecules where a core protein is extensively decorated with many glycosaminoglycan (GAG) chains covalently attached. They are characterized by being mostly carbohydrate by weight (often 95% carbohydrate, 5% protein).
Key Characteristics & Functions:
What they are: Proteins that have relatively short, branched carbohydrate chains (oligosaccharides) covalently attached to them. Unlike proteoglycans, the protein component is usually dominant, with carbohydrate content typically ranging from 1% to 15% by weight.
Key Characteristics & Functions:
| Carbohydrate | Type | Monosaccharides Involved | Glycosidic Bond(s) | Reducing? (Practical) | Function/Notes |
|---|---|---|---|---|---|
| Sucrose | Disaccharide | Glucose + Fructose | α-1,2 | No | Table sugar; transport in plants; easily digestible. |
| Lactose | Disaccharide | Galactose + Glucose | β-1,4 | Yes | Milk sugar; digestion requires lactase; common intolerance. |
| Maltose | Disaccharide | Glucose + Glucose | α-1,4 | Yes | Intermediate in starch digestion; brewing. |
| Starch | Homopolysaccharide | Glucose (Amylose & Amylopectin) | α-1,4; α-1,6 (branches) | Weakly/No | Energy storage in plants; digestible by humans. |
| Glycogen | Homopolysaccharide | Glucose | α-1,4; α-1,6 (branches) | Weakly/No | Energy storage in animals (liver, muscle); highly branched for rapid glucose release. |
| Cellulose | Homopolysaccharide | Glucose | β-1,4 | No | Structural support in plants; indigestible fiber for humans. |
| Chitin | Homopolysaccharide | N-acetylglucosamine | β-1,4 | No | Structural in fungi/arthropods (exoskeletons); indigestible. |
| Hyaluronic Acid | Heteropolysaccharide | D-glucuronic acid + N-acetylglucosamine | Varied, β-linkages | No | Lubrication, shock absorption, tissue hydration; non-sulfated GAG. |
| Heparin | Heteropolysaccharide | D-glucuronic acid/L-iduronic acid + N-sulfo-D-glucosamine | Varied, α- and β-linkages | No | Anticoagulant; highly sulfated. |
| Chondroitin Sulfate | Heteropolysaccharide | D-glucuronic acid + N-acetyl-D-galactosamine-sulfate | Varied, β-linkages | No | Cartilage structure and resilience. |
| Proteoglycans | Glycoconjugate | Protein + many GAG chains | Covalent protein-GAG link | No | Major ECM component; hydration, compression resistance (e.g., Aggrecan in cartilage). |
| Glycoproteins | Glycoconjugate | Protein + few, branched oligosaccharides | Covalent protein-sugar link | No | Cell recognition, signaling, immune function, receptors (e.g., blood group antigens). |
Test your knowledge with these 51 questions.
Question 1/51
Here are your results, .
Your Score
48/51
94%
Test your knowledge with these 30 questions.
Question 1/30
Here are your results, .
Your Score
28/30
93%
Test your knowledge with these 40 questions.
Question 1/40
Here are your results, .
Your Score
38/40
95%
Test your knowledge with these 40 questions.
Question 1/40
Here are your results, .
Your Score
38/40
95%
Abnormal hemoglobin refers to any variant of the hemoglobin molecule that deviates from the normal adult hemoglobin (HbA) in its primary amino acid sequence, structure, or quantity, leading to impaired function or stability. These abnormalities can result in a range of clinical conditions, collectively known as hemoglobinopathies, affecting the red blood cells' ability to effectively transport oxygen.
Abnormal hemoglobins are broadly classified based on the nature of their underlying molecular defect:
(Qualitative Defects): Involve a change in the amino acid sequence of a globin chain, often from a point mutation. This results in an abnormal protein. Examples: HbS, HbC, HbE.
(Quantitative Defects): Involve reduced or absent production of a structurally normal globin chain due to gene deletions or mutations. This leads to a chain imbalance. Examples: α-thalassemia, β-thalassemia.
Structural variants where an amino acid substitution destabilizes the molecule, causing it to precipitate and lead to chronic hemolysis and Heinz body formation.
Structural variants where amino acid changes affect allosteric properties, altering the ability to bind and release oxygen, leading to polycythemia or cyanosis.
Structural hemoglobinopathies are characterized by the synthesis of an abnormal globin chain due to a mutation in the globin gene.
Molecular Basis: β6Glu→Val (Glutamate to Valine).
Impact: Creates a hydrophobic patch, leading to polymerization of deoxygenated HbS.
Syndrome: Sickle Cell Disease. Rigid sickled cells cause vaso-occlusion (pain crises) and chronic hemolytic anemia.
Molecular Basis: β6Glu→Lys (Glutamate to Lysine).
Impact: Reduced solubility causes HbC to crystallize within RBCs.
Syndrome: HbC Disease. Mild chronic hemolytic anemia, splenomegaly, and characteristic "target cells" on blood smear.
Molecular Basis: β26Glu→Lys (Glutamate to Lysine).
Impact: Creates an alternative mRNA splice site, causing a mild quantitative defect (thalassemic effect).
Syndrome: Mild microcytic anemia. Clinically significant when co-inherited with β-thalassemia.
Thalassemias are characterized by a reduced rate of synthesis or absence of one or more of the globin chains, leading to an imbalance in the production of α and β globin chains. The individual globin chains produced are structurally normal.
Genetic Defect: Deletion of one or more of the four α-globin genes on chromosome 16.
Pathology: Excess β or γ chains form unstable tetramers (HbH, Hb Barts) that are poor oxygen carriers, leading to hemolysis and ineffective erythropoiesis.
Spectrum: Severity depends on the number of genes deleted, ranging from a silent carrier (1 gene) to fatal hydrops fetalis (4 genes).
Genetic Defect: Point mutations in the two β-globin genes on chromosome 11, reducing (β+) or eliminating (β0) synthesis.
Pathology: Excess α-chains are highly insoluble and precipitate in RBC precursors, causing severe ineffective erythropoiesis and hemolysis.
Spectrum: Ranges from asymptomatic trait (minor) to transfusion-dependent anemic (major).
These are structural hemoglobin variants where amino acid substitutions alter the allosteric regulation of oxygen binding and release.
Mechanism: Mutations stabilize the R (oxygenated) state, making it harder to release O₂ to tissues.
Presentation (Polycythemia): Tissue hypoxia stimulates erythropoietin, leading to increased red blood cell production (erythrocytosis).
Examples: Hb Chesapeake, Hb Suresnes.
Mechanism: Mutations stabilize the T (deoxygenated) state, causing premature O₂ release.
Presentation (Cyanosis): Higher levels of deoxygenated Hb in arterial blood cause a bluish discoloration of the skin, though O₂ delivery is adequate.
Examples: Hb Kansas, Hb Beth Israel.
The diagnosis of abnormal hemoglobin disorders relies on a combination of clinical evaluation and specialized laboratory tests:
Therapeutic approaches vary widely depending on the specific abnormal hemoglobin and its severity:
A 2-year-old boy from Mukono district presents with recurrent episodes of severe bone pain (hands, feet, and sternum pain), jaundice, and fatigue for 3 days.
Laboratory findings reveal:
A diagnosis of Vaso-occlusive crisis, and severe anaemia in Sickle Cell Disease was made.
This part requires a detailed breakdown of the specific molecular error in the patient's haemoglobin protein, focusing on the identity of the amino acids and the genetic origin of the mistake.
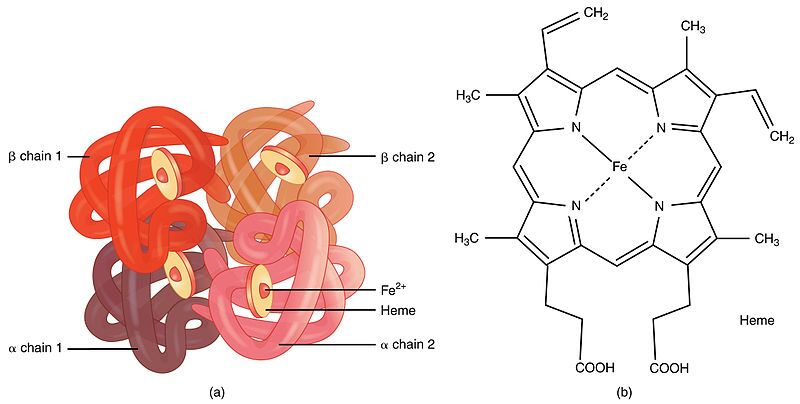
First, it's important to understand what haemoglobin is. Haemoglobin is the primary protein found within red blood cells (erythrocytes) and its main function is to transport oxygen from the lungs to the body's tissues. It is a large, complex protein with a quaternary structure, meaning it is composed of multiple polypeptide subunits. A normal adult haemoglobin molecule (HbA) is a tetramer, consisting of four chains: two identical alpha (α)-globin chains and two identical beta (β)-globin chains. The genetic defect in sickle cell disease specifically affects the gene that provides the instructions for the beta-globin chain.

The defining molecular event in sickle cell disease is a single amino acid substitution at a precise location within the beta-globin polypeptide chain.
In a person with normal adult haemoglobin (HbA), the amino acid at the sixth position from the beginning (the N-terminus) of the beta-globin chain is Glutamic Acid (abbreviated as Glu or E).
In this patient with sickle cell disease, the haemoglobin is abnormal (called HbS). At that exact same sixth position, the Glutamic Acid has been replaced by the amino acid Valine (abbreviated as Val or V).
This single change, Glu6Val, is the sole cause of the disease.

The severity of this substitution is due to the drastically different chemical "personalities" of the R-groups (side chains) of Glutamic Acid and Valine. This position is on the outer surface of the protein, where it is exposed to the watery environment inside the red blood cell.
| Amino Acid | Chemical Class & Properties | Behavior in Water |
|---|---|---|
| Glutamic Acid (Normal) | Its side chain contains a carboxyl group (`-CH₂-CH₂-COOH`). At the neutral pH inside a red blood cell (~7.4), this group loses a proton and becomes negatively charged (`-COO⁻`). Therefore, it is an acidic, polar, and charged amino acid. | Because it is charged and polar, Glutamic Acid is hydrophilic ("water-loving"). It forms favorable interactions with polar water molecules and is perfectly stable on the protein's surface. |
| Valine (Mutant) | Its side chain is an isopropyl group (`-CH(CH₃)₂`), which is a small, branched structure made only of carbon and hydrogen. These bonds are nonpolar. Therefore, Valine is a nonpolar, aliphatic, and neutral amino acid. | Because it is nonpolar, Valine is hydrophobic ("water-fearing"). It is thermodynamically unfavorable for this "oily" side chain to be exposed to water. It will seek to interact with other nonpolar groups to hide from the aqueous environment. |
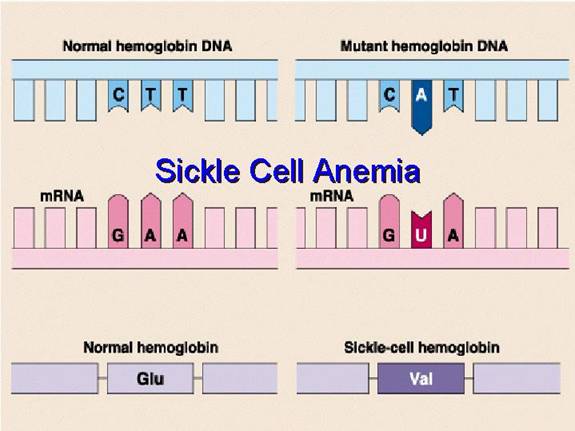
This amino acid error originates from a single change in the DNA sequence of the beta-globin gene. This type of mutation is called a point mutation, specifically a missense mutation because it results in a codon that codes for a different amino acid.
Therefore, a single DNA base change leads to a single mRNA codon change, which in turn leads to the single, catastrophic amino acid substitution that defines sickle cell disease.
This section explains the step-by-step process of how the single Glu6Val substitution causes the haemoglobin to malfunction and leads to the patient's observed symptoms.

The key event is the behavior of HbS when it is in the deoxygenated state. In the oxygenated state (in the lungs), HbS functions almost normally as an oxygen carrier.
Shape Distortion: These long, stiff haemoglobin polymers grow to be longer than the diameter of the red blood cell itself. They physically push against the cell membrane from the inside, distorting the cell from its normal, flexible biconcave disc shape into a rigid, elongated, crescent or "sickle" shape.
Loss of Deformability: This sickling process causes a dramatic loss of the cell's flexibility. It becomes hard and unable to deform. This process is initially reversible if the cell becomes reoxygenated, but repeated sickling events cause permanent membrane damage, leading to irreversibly sickled cells.
The physical properties of these sickled cells are directly responsible for the patient's symptoms:
Knowing that the core problem is a hydrophobic amino acid causing polymerization allows for the design of targeted therapies.
This approach aims to reduce the relative concentration of the problematic HbS.
This is the most direct chemical approach, aiming to stop the Valine from interacting with its target.
This is the most fundamental approach, aiming to fix the DNA instruction so the correct amino acid is made.
Test your knowledge with these 25 questions.
Question 1/25
Here are your results, .
Your Score
23/25
92%
Test your knowledge with these 51 questions.
Question 1/51
Here are your results, .
Your Score
48/51
94%