White blood Cell: Physiology
White Blood Cells (Leukocytes)
White blood cells (WBCs), also known as leukocytes, are a diverse group of immune cells that circulate in the blood and lymphatic system. Unlike red blood cells, they are complete cells, possessing a nucleus and other organelles, and their primary function is to defend the body against infection and disease. They are generally much less numerous than RBCs.
Leukocytes are broadly classified into two main categories based on the presence or absence of visible granules in their cytoplasm when stained with Romanowsky stains (like Wright's or Giemsa):
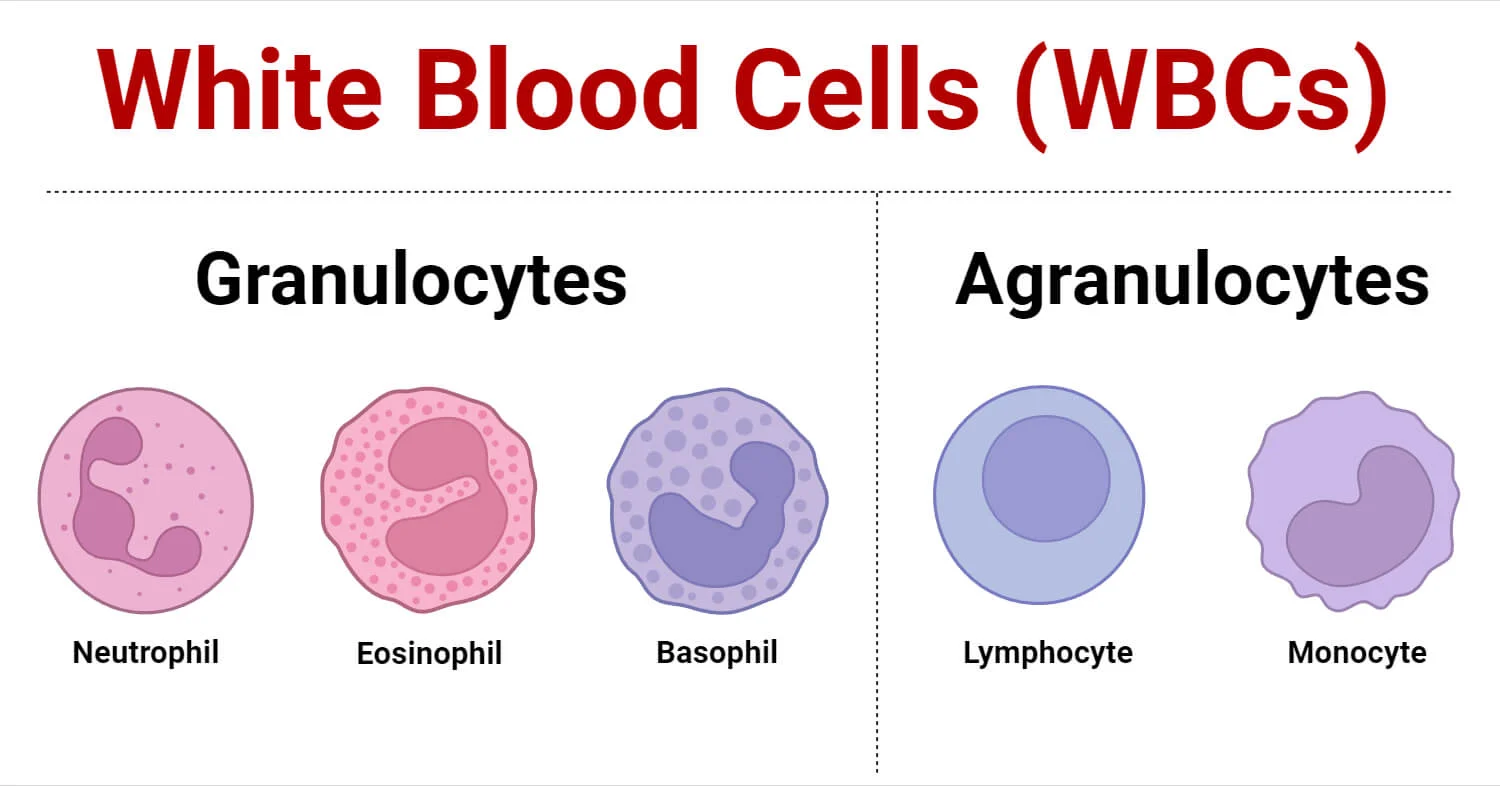
I. Granulocytes
These cells have prominent cytoplasmic granules that contain various enzymes and antimicrobial substances. They also have lobed nuclei.
1. Neutrophils
Polymorphonuclear Leukocytes - PMNs
Morphology
- Nucleus: Multi-lobed nucleus (usually 2-5 lobes) connected by thin strands of chromatin. Lobes increase with age.
- Cytoplasm: Fine, pale lilac or pinkish-tan granules; typically very faint.
- Size: 10-14 µm.
Key Features
- Abundance: Most numerous (50-70%).
- Key Characteristic: "First responders" against bacteria.
- Phagocytosis: Rapid responders to bacterial/fungal infections. First to arrive at inflammation.
- Destroy Pathogens: Granules contain lysosomal enzymes, defensins, and antimicrobial agents.
- Formation of Pus: Dead neutrophils + debris + bacteria form pus.
2. Eosinophils
Morphology
- Nucleus: Bi-lobed nucleus, resembling eyeglasses or headphones.
- Cytoplasm: Large, coarse, distinct red-orange granules.
- Size: 12-17 µm.
Key Features
- Abundance: Relatively uncommon (1-4%).
- Key Characteristic: Associated with parasites and allergies.
- Parasitic Infections: Effective against multicellular parasites (worms) via toxic granule release.
- Allergic Reactions: Modulate responses by releasing antihistamines. Accumulate in asthma/hay fever.
3. Basophils
Morphology
- Nucleus: Bi-lobed/S-shaped, often obscured by granules.
- Cytoplasm: Large, coarse, distinct dark blue-purple granules containing histamine and heparin.
- Size: 10-14 µm.
Key Features
- Abundance: Rarest WBC (0.5-1%).
- Key Characteristic: Severe allergic reactions, histamine release.
- Allergic/Inflammatory Responses: Release histamine (vasodilator) and heparin (anticoagulant).
- Similar to Mast Cells: Share functional similarities but are distinct cells.
II. Agranulocytes
These cells have few or no visible granules in their cytoplasm. Their nuclei are typically non-lobed or kidney-shaped.
1. Lymphocytes
Morphology
- Nucleus: Large, round, densely stained; occupies most of the cell.
- Cytoplasm: Scant, light blue rim; few/no granules.
- Size: Variable; small (7-9 µm) most common.
Key Features
- Abundance: Second most numerous (20-40%).
- Key Characteristic: Immune "memory" and specific defense.
- T Lymphocytes (T cells): Cell-mediated immunity (attack virus-infected/cancer cells).
- B Lymphocytes (B cells): Humoral immunity (produce antibodies). Differentiate into plasma cells.
- NK Cells: Rapid response to infected/tumor cells (Innate immunity).
2. Monocytes
Morphology
- Nucleus: Large, kidney or horse-shoe shaped; lighter stain.
- Cytoplasm: Abundant, pale gray-blue ("ground-glass").
- Size: Largest WBC (14-20 µm).
Key Features
- Abundance: 2-8%.
- Key Characteristic: Precursors to macrophages, "big eaters."
- Macrophages: Circulate briefly then migrate to tissues to differentiate into macrophages.
- Phagocytosis: Engulf bacteria, debris, old RBCs ("clean-up crew").
- Antigen Presentation: Present antigens to lymphocytes.
- Chronic Inflammation: Crucial role.
Summary Table of WBC Types
| WBC Type | Granules (Staining) | Nucleus Morphology | Abundance | Primary Function |
|---|---|---|---|---|
| Neutrophil | Fine, pale lilac | 2-5 lobes, polymorphous | 50-70% | Phagocytosis of bacteria/fungi (first responders) |
| Eosinophil | Large, red-orange | Bi-lobed | 1-4% | Allergic reactions, parasitic infections |
| Basophil | Large, dark blue-purple | Bi-lobed, often obscured | 0.5-1% | Allergic reactions (histamine), inflammation |
| Lymphocyte | None/scant | Large, round, dense | 20-40% | Specific immunity (T/B cells), memory |
| Monocyte | None/fine dust-like | Kidney-shaped, horse-shoe | 2-8% | Phagocytosis (macrophages), antigen presentation |
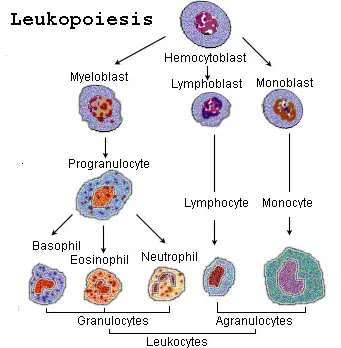
Process of Leukopoiesis
Leukopoiesis is the process of white blood cell (WBC) production, occurring primarily in the red bone marrow. Unlike erythropoiesis, which is mainly stimulated by erythropoietin, leukopoiesis involves a broader array of growth factors called colony-stimulating factors (CSFs) and interleukins (ILs) that guide the differentiation of hematopoietic stem cells into the various leukocyte lineages.
I. Hematopoietic Stem Cells (HSCs) and Lineage Commitment
All blood cells originate from pluripotent Hematopoietic Stem Cells (HSCs) in the red bone marrow. These HSCs differentiate into two major progenitor cell lines:
Common Myeloid Progenitor (CMP)
Gives rise to granulocytes (neutrophils, eosinophils, basophils), monocytes, red blood cells, and platelets.
Common Lymphoid Progenitor (CLP)
Gives rise to lymphocytes (T cells, B cells, NK cells).
II. Myelopoiesis (Granulocytes & Monocytes)
This is the pathway from the CMP to mature granulocytes and monocytes.
Pathway: Common Myeloid Progenitor (CMP) → Granulocyte-Monocyte Progenitor (GMP) (A bipotential progenitor).
A. Granulocyte Development (Neutrophil, Eosinophil, Basophil)
First morphologically recognizable precursor. Large cell, prominent nucleus, fine chromatin, basophilic cytoplasm, no granules.
Larger than myeloblast. Prominent primary (azurophilic) granules (dark purple).
Beginning of specific granule synthesis (neutrophilic, eosinophilic, or basophilic). Nucleus becomes more kidney-shaped. Last stage capable of mitosis.
Nucleus indented (kidney-bean shaped). No longer capable of mitosis.
Nucleus elongated and curved (Band or "C" shape), not fully segmented. Released in infection ("left shift").
Nucleus segmented (multi-lobed for neutrophils, bi-lobed for eosinophils/basophils).
B. Monocyte Development
- Monoblast: Precursor, similar to myeloblast but committed to monocytic lineage.
- Promonocyte: Large cell with indented nucleus, somewhat basophilic cytoplasm.
- Monocyte: Mature cell released into bloodstream. Circulates briefly before migrating to tissues to become a macrophage or dendritic cell.
III. Lymphopoiesis (Lymphocytes)
Pathway from Common Lymphoid Progenitor (CLP) to mature lymphocytes.
Stages:
- Lymphoblast: First recognizable precursor. Large nucleus, scant cytoplasm.
- Prolymphocyte: Slightly smaller, less prominent nucleolus.
- Lymphocyte: Mature cells released into circulation.
Maturation Sites:
- B Lymphocytes: Mature in Bone Marrow → migrate to lymph nodes/spleen.
- T Lymphocytes: Migrate from marrow to Thymus to mature and undergo selection.
- NK Cells: Mature in marrow and secondary lymphoid organs.
IV. Regulation: Colony-Stimulating Factors (CSFs) and Interleukins (ILs)
Leukopoiesis is tightly regulated by a complex network of signaling molecules (glycoproteins) acting as growth factors.
Colony-Stimulating Factors
GM-CSF (Granulocyte-Macrophage CSF): Stimulates production of granulocytes and monocytes/macrophages from myeloid progenitors.
G-CSF (Granulocyte CSF): Primarily stimulates production and maturation of neutrophils.
Clinical: Used to boost neutrophil counts in neutropenic patients.
M-CSF (Macrophage CSF): Promotes differentiation of monocytes into macrophages.
Interleukins (ILs) & Others
IL-3: Multilineage CSF; stimulates growth of various hematopoietic stem cells (myeloid & lymphoid).
IL-5: Crucial for growth, differentiation, and activation of eosinophils.
IL-7: Essential for development of B and T lymphocytes.
IL-6: Involved in immune responses; stimulates HSCs.
Stem Cell Factor (SCF / c-kit ligand): Important for survival and proliferation of early HSCs.
Summary of Leukopoiesis
- Originates from HSCs in red bone marrow.
- Differentiates into CMP (Myeloid) and CLP (Lymphoid).
- Myeloid Lineage: Produces granulocytes and monocytes (regulated by GM-CSF, G-CSF, M-CSF, ILs).
- Lymphoid Lineage: Produces lymphocytes (regulated by IL-7).
- Mature T cells undergo further maturation in the thymus.
Common Disorders Associated with White Blood Cells
Disorders involving white blood cells can range from simple numerical changes (too many or too few) to malignant transformations of the cells themselves. These conditions often have significant impacts on the body's immune function and overall health.
I. Quantitative Disorders (Changes in Number)
These involve an abnormal increase or decrease in the total number of WBCs, or specific types of WBCs, in the peripheral blood.
1. Leukocytosis
Definition: An increase in the total white blood cell count above the normal range (>11,000 WBCs/µL).
Causes
- Infection: Most common (bacterial, viral, fungal, parasitic).
- Inflammation: Non-infectious (autoimmune, burns).
- Stress: Physical/emotional (cortisol mobilizes WBCs).
- Medications: Steroids, G-CSF.
- Leukemia: Malignant proliferation.
Specific Types
- Neutrophilia: Bacterial infections, inflammation.
- Lymphocytosis: Viral infections (Mono), chronic infections.
- Eosinophilia: Parasites, allergies, skin conditions.
- Basophilia: Rare, myeloproliferative disorders.
- Monocytosis: Chronic infections (TB), autoimmune, recovery phase.
2. Leukopenia
Definition: A decrease in the total white blood cell count below the normal range (<4,000 WBCs/µL).
Causes
- Marrow Suppression: Chemo, radiation, drugs, aplastic anemia, viral (HIV).
- Autoimmune: Lupus (SLE), Rheumatoid Arthritis.
- Splenic Sequestration: Enlarged spleen traps WBCs.
- Overwhelming Infection: Used up faster than produced (sepsis).
Specific Types
- Neutropenia: High susceptibility to bacterial/fungal infection. Most clinically significant.
- Lymphopenia: Immunodeficiency (HIV/AIDS), steroids, radiation.
II. Qualitative Disorders (Function/Morphology)
Abnormalities in structure or function, even if numbers are normal.
Inherited condition where neutrophils have hyposegmented (bilobed/unlobed) nuclei, but function is usually normal.
Rare genetic disorder with giant, abnormal granules in phagocytes/lymphocytes. Impaired phagocytosis → increased infections.
Phagocytes cannot produce reactive oxygen species (e.g., superoxide) effectively, impairing killing of certain bacteria/fungi.
III. Malignant Disorders (Cancers of WBCs)
Uncontrolled proliferation of abnormal WBCs or precursors.
1. Leukemia
Definition: Cancers originating in bone marrow/lymphoid tissues characterized by uncontrolled proliferation of abnormal, immature WBCs (blasts) that accumulate in marrow and spill into blood.
Classification:
- Acute vs. Chronic:
- Acute: Rapid onset, highly immature cells (blasts). (AML, ALL).
- Chronic: Slower onset, more mature abnormal cells. (CML, CLL).
- Myeloid vs. Lymphoid:
- Myeloid: Granulocytes, monocytes, RBCs, platelets.
- Lymphoid: Lymphocytes.
Symptoms: Marrow failure (anemia, bleeding, infection) and organ infiltration (lymphadenopathy, splenomegaly).
2. Lymphoma
Definition: Cancers originating in the lymphatic system (nodes, spleen, thymus). Typically forms solid tumors rather than circulating widely initially.
Main Types:
- Hodgkin Lymphoma (HL): Presence of Reed-Sternberg cells. Orderly spread.
- Non-Hodgkin Lymphoma (NHL): Diverse group (B, T, or NK cells). More common, varied spread.
Symptoms: Painless lymphadenopathy, "B symptoms" (fever, night sweats, weight loss), fatigue, pruritus.
3. Multiple Myeloma
Definition: Cancer of plasma cells (differentiated B cells) proliferating in bone marrow.
Key Features: Production of large amounts of abnormal antibodies (M-protein), bone lesions (pain/fractures), hypercalcemia, kidney failure, anemia.
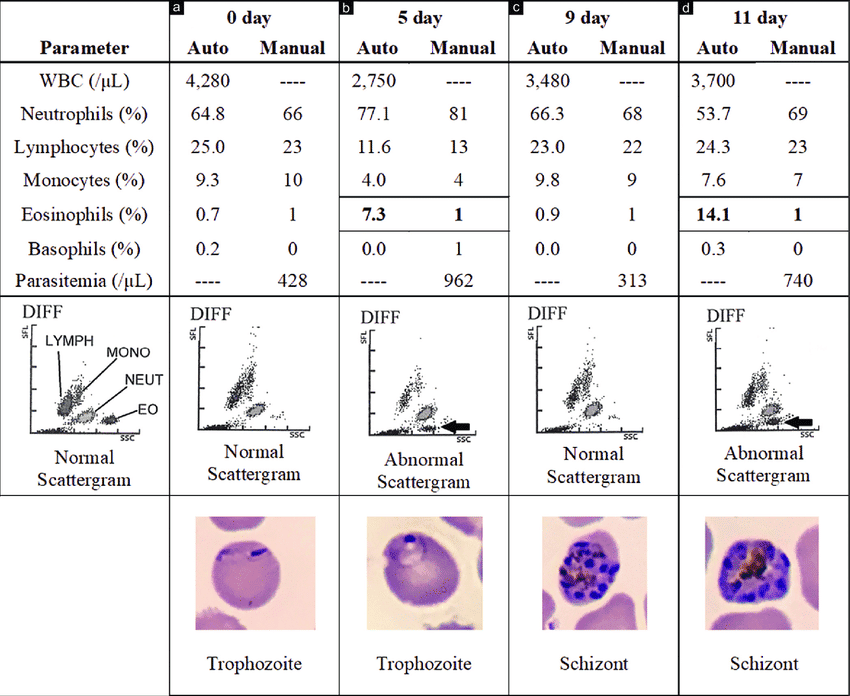
Clinical Significance of a Differential White Blood Cell Count
A complete blood count (CBC) with differential is a routine blood test that provides valuable information about the different types of white blood cells (WBCs) present in a patient's blood. It not only gives the total WBC count but also the percentage and absolute number of each of the five main types of leukocytes. This "differential" count is a powerful diagnostic tool, as specific patterns of changes in WBC populations can indicate various underlying conditions.
I. How a Differential WBC Count is Performed
1. Automated Counters
Modern hematology analyzers quickly count and classify thousands of cells based on their size, granularity, and nuclear complexity using light scattering and electrical impedance.
2. Manual Differential
If the automated count is abnormal, or if there is a concern for atypical cells, a technologist examines a stained blood smear under a microscope to visually identify abnormal morphologies.
II. Clinical Significance of Changes in Specific WBC Types
Understanding normal ranges and causes of increases (–philia/-cytosis) and decreases (–penia) is critical.
1. Neutrophils
ANC: 2,500-7,000/µL
- Significance: Strong indicator of acute bacterial infections. Also seen in inflammation (appendicitis), tissue necrosis (MI), physical stress, corticosteroids.
- "Left Shift": Presence of increased immature neutrophils (bands, metamyelocytes). Indicates marrow is rapidly releasing cells to fight severe infection.
- Significance: Increases susceptibility to severe bacterial/fungal infections.
- Causes: Marrow suppression (chemo/radiation), viral infections (flu, HIV), aplastic anemia, autoimmune.
2. Lymphocytes
ALC: 1,000-4,000/µL
- Significance: Often associated with viral infections (Mono, hepatitis). Also chronic bacterial (TB) and lymphoid leukemia.
- Atypical Lymphocytes: Large, irregular cells seen in viral infections.
- Significance: Indicates impaired immune function.
- Causes: Immunodeficiency (HIV/AIDS), steroids, radiation, stress, autoimmune.
3. Monocytes
AMC: 100-800/µL
Monocytosis (Increased)Significance: Suggests chronic inflammation or chronic infection (TB, endocarditis, fungal). Seen in recovery phase of acute infection. Indicates effort to clear debris.
4. Eosinophils
AEC: 50-400/µL
EosinophiliaSignificance: Highly indicative of allergic reactions (asthma/hay fever) and parasitic infections (worms).
5. Basophils
ABC: 20-100/µL
BasophiliaSignificance: Rare. Seen in allergic reactions and myeloproliferative disorders (CML).
III. Interpreting the Differential in Clinical Context
A single abnormal value is rarely diagnostic on its own. It must be interpreted with symptoms, medical history, other CBC parameters, trends, and lab tests.
Examples of Differential Interpretation:
-
High Total WBC + Neutrophilia + Left Shift:
Likely acute bacterial infection. -
Normal/High WBC + Lymphocytosis + Atypical Lymphocytes:
Suggests acute viral infection (e.g., infectious mononucleosis). -
Elevated Eosinophils + Rash/Itching:
Points towards allergy or parasitic infection. -
High Total WBC + Significant Immature Blasts:
Suggests leukemia. -
Neutropenia + Fever:
Medical emergency due to high risk of severe infection.
The differential white blood cell count is an indispensable tool in clinical medicine, guiding clinicians towards appropriate diagnostic workups and treatment strategies.
White Blood Cells Quiz
Test your knowledge with these 30 questions.
White Blood Cells Quiz
Question 1/30
Quiz Complete!
Here are your results, .
Your Score
27/30
90%