Glycogenolysis & Glycogenesis
Glycogenolysis
Glycogenolysis is the biochemical process by which glycogen, a stored form of glucose, is broken down into glucose-1-phosphate and then subsequently converted to glucose or glucose-6-phosphate. The suffix "-lysis" means "to break down," so it literally means "breaking down glycogen."
Glycogen itself is a highly branched polysaccharide composed of glucose units. It serves as the primary storage form of glucose in animals. In humans, it is predominantly stored in the liver and skeletal muscles.
Purpose
The primary purpose of glycogenolysis is to mobilize stored glucose to meet the body's immediate energy needs, particularly to maintain stable blood glucose levels and provide fuel for muscle contraction.
-
Maintenance of Blood Glucose Homeostasis (Liver Glycogenolysis):
- The liver is crucial for regulating blood glucose. When blood glucose levels drop (e.g., during fasting or intense exercise), the liver breaks down its glycogen stores.
- The glucose-6-phosphate produced in the liver can be dephosphorylated to free glucose and then released into the bloodstream, supplying fuel to other tissues like the brain and red blood cells.
-
Energy for Muscle Contraction (Muscle Glycogenolysis):
- Skeletal muscles also store glycogen, but unlike liver glycogen, it is primarily used to fuel the muscle's own activity.
- During exercise, muscle glycogen is broken down to glucose-6-phosphate, which then enters glycolysis to produce ATP directly within the muscle cells. Muscle cells lack the enzyme glucose-6-phosphatase, so they cannot release free glucose into the bloodstream.
Location
Glycogenolysis primarily occurs in two major tissues in the human body:
Liver
- Primary Role: The liver is the main organ responsible for maintaining blood glucose homeostasis.
- Capacity: The liver stores a significant amount of glycogen (up to 6-8% of its wet weight, or about 100-120 grams in an adult).
- This is important for absolutely glucose-dependent cells like neurons, RBCs, and the renal medulla.
- Mechanism: When blood glucose drops, liver glycogen is broken down. The resulting glucose-6-phosphate is dephosphorylated by the enzyme glucose-6-phosphatase to free glucose, which is then released into the bloodstream.
- Regulation: Liver glycogenolysis is highly regulated by hormones such as glucagon (released during low blood glucose) and epinephrine (released during stress).
Skeletal Muscles
- Primary Role: Muscle glycogen serves as a readily available fuel source for the muscle itself during physical activity.
- Capacity: Skeletal muscles collectively store a larger total amount of glycogen than the liver (about 1-2% of muscle wet weight, or about 300-500 grams in an adult).
- Mechanism: During exercise, muscle glycogen is broken down to glucose-6-phosphate, which directly enters glycolysis within the muscle cell to produce ATP.
- Key Difference from Liver: Muscle cells lack the enzyme glucose-6-phosphatase. This means muscle glycogen cannot be used to directly replenish blood glucose. The glucose-6-phosphate is "trapped" within the muscle cell.
- Regulation: Muscle glycogenolysis is primarily regulated by epinephrine (during "fight or flight" responses) and by AMP (which signals a low energy state).
Key Enzymes
The breakdown of glycogen is a well-orchestrated process involving a few critical enzymes working in sequence. These enzymes ensure that glucose units are efficiently released from the glycogen molecule.
The three main enzymes (or enzyme complexes) are:
- Glycogen Phosphorylase
- Debranching Enzyme (which has two enzymatic activities)
- Phosphoglucomutase
Let's look at each one:
1. Glycogen Phosphorylase
- Function: This is the primary enzyme responsible for breaking down glycogen. It catalyzes the phosphorolysis (breaking a bond using inorganic phosphate, not water) of the
α(1,4)glycosidic bonds that link glucose units. - Mechanism: It removes glucose units one by one from the non-reducing ends of the glycogen molecule. The bond is broken by the addition of inorganic phosphate (Pᵢ), yielding glucose-1-phosphate.
- Limitation: Glycogen phosphorylase cannot break the
α(1,6)glycosidic bonds at the branch points. It stops cleaving when it reaches about four glucose residues away from a branch point, leaving behind a "limit dextrin."
2. Debranching Enzyme (Glycogen Debranching Enzyme)
Since glycogen phosphorylase cannot handle the branch points, this specialized enzyme complex is required. It has two distinct catalytic activities:
a. Oligo-α(1,4)-α(1,4)-glucantransferase activity (Transferase activity):
- Function: This activity transfers a block of three glucose residues from a branch to a non-reducing end of another chain. It forms a new
α(1,4)bond, making the chain longer and available for further action by glycogen phosphorylase.
b. Amylo-α(1,6)-glucosidase activity (Glucosidase activity):
- Function: After the transferase activity, this activity hydrolyzes the single remaining glucose residue at the
α(1,6)branch point, releasing it as free glucose (not glucose-1-phosphate). - Significance: This is the only step in glycogenolysis that directly produces free glucose (about 8% of the glucose from glycogen is released this way).
3. Phosphoglucomutase
- Function: This enzyme is responsible for interconverting glucose-1-phosphate and glucose-6-phosphate.
- Mechanism: Glycogen phosphorylase produces glucose-1-phosphate. For this glucose to enter glycolysis (as glucose-6-phosphate) or to be released into the bloodstream (as free glucose in the liver), it first needs to be converted. Phosphoglucomutase catalyzes this reversible isomerization.
Summary of Enzyme Action:
- Glycogen Phosphorylase removes glucose units as glucose-1-phosphate from the linear parts of glycogen.
- Debranching Enzyme "cleans up" the branch points: its transferase activity moves most of the branch, and its glucosidase activity releases the final branched glucose as free glucose.
- Phosphoglucomutase converts the glucose-1-phosphate into glucose-6-phosphate, which is the entry point for further metabolism.
Steps of the Glycogenolysis Pathway
Here's a step-by-step breakdown of how glycogen is degraded to release glucose units, incorporating the enzymes we just discussed.
Overall Goal: To convert glycogen into individual glucose units that can be used for energy or released into the bloodstream.
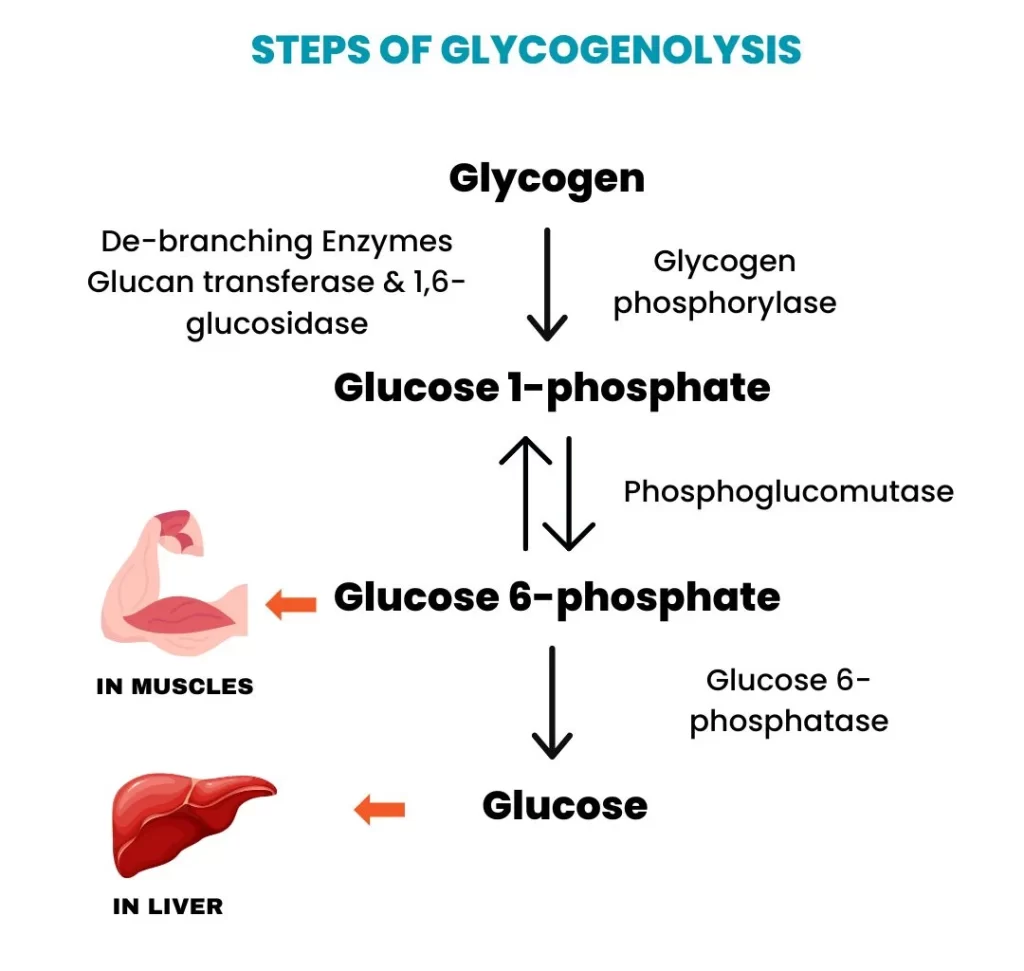
Step 1: Phosphorolytic Cleavage of α(1,4) Glycosidic Bonds
- Enzyme: Glycogen Phosphorylase
- Action: Begins acting on the non-reducing ends of the glycogen molecule, cleaving the
α(1,4)glycosidic bonds. - Product: Each cleavage releases a molecule of glucose-1-phosphate (G1P). This process is called phosphorolysis because inorganic phosphate (Pᵢ) is used to break the bond.
- Limitation: The enzyme stops when it reaches approximately four glucose residues away from an
α(1,6)branch point, leaving a "limit dextrin."
Step 2: Remodeling of the Glycogen Molecule at Branch Points
- Enzyme: Debranching Enzyme
- Action: The debranching enzyme resolves the limit dextrin structure:
- Transferase Activity (Oligo-α(1,4)-α(1,4)-glucantransferase): Transfers a block of three glucose residues from the branch and reattaches them to a nearby non-reducing end via an
α(1,4)bond. - Glucosidase Activity (Amylo-α(1,6)-glucosidase): Hydrolyzes the single remaining
α(1,6)bond, releasing the glucose residue as free glucose.
- Transferase Activity (Oligo-α(1,4)-α(1,4)-glucantransferase): Transfers a block of three glucose residues from the branch and reattaches them to a nearby non-reducing end via an
- Result: Once the branch point is removed, glycogen phosphorylase can resume its action on the now-longer unbranched chain.
Step 3: Isomerization of Glucose-1-Phosphate to Glucose-6-Phosphate
- Enzyme: Phosphoglucomutase
- Action: The vast majority of glucose units released are in the form of G1P. For this to be used, it must be converted to glucose-6-phosphate (G6P). Phosphoglucomutase catalyzes this reversible isomerization.
- Significance:
- In Muscle: G6P directly enters the glycolysis pathway to produce ATP.
- In Liver: G6P can enter glycolysis or be dephosphorylated to free glucose for release into the bloodstream.
Step 4: Dephosphorylation of Glucose-6-Phosphate (Liver Specific)
- Enzyme: Glucose-6-phosphatase
- Location: Primarily found in the liver, but absent in muscle.
- Action: Removes the phosphate group from G6P, producing free glucose.
- Significance: This free glucose can then be transported out of the liver cell and into the bloodstream, raising blood glucose levels.
Simplified Flow:
Glycogen (n residues)
↓ (Glycogen Phosphorylase)
Glucose-1-Phosphate (G1P) + Glycogen (n-1 residues)
(Repeat for α(1,4) bonds)
At branch points:
Limit Dextrin
↓ (Debranching Enzyme - Transferase)
Lengthened α(1,4) chain + single α(1,6) linked glucose
↓ (Debranching Enzyme - Glucosidase)
Free Glucose
Back to G1P:
Glucose-1-Phosphate (G1P)
↓ (Phosphoglucomutase)
Glucose-6-Phosphate (G6P)
In Liver Only:
Glucose-6-Phosphate (G6P)
↓ (Glucose-6-phosphatase)
Free Glucose → Bloodstream
Products
The primary products of glycogenolysis depend on where the process is occurring (liver vs. muscle) and the specific enzymes involved.
Glucose-1-Phosphate (G1P)
- This is the main product of the action of glycogen phosphorylase, which cleaves the
α(1,4)glycosidic bonds. - It represents the vast majority (about 90-92%) of the glucose units released from glycogen.
Glucose-6-Phosphate (G6P)
- G1P is readily converted to G6P by phosphoglucomutase.
- In Muscle: G6P is the final form of glucose released from muscle glycogen and immediately enters glycolysis to produce ATP for muscle contraction. It cannot be converted to free glucose in muscle.
- In Liver: G6P is an intermediate that can either enter glycolysis or be further processed to free glucose for release into the bloodstream.
Free Glucose
- From Debranching Enzyme: A small amount of free glucose (about 8-10%) is produced directly by the amylo-α(1,6)-glucosidase activity of the debranching enzyme, which hydrolyzes the
α(1,6)branch points. - From Glucose-6-phosphatase (Liver-Specific): In the liver, the enzyme glucose-6-phosphatase dephosphorylates G6P to produce free glucose. This free glucose is then released into the bloodstream to maintain blood glucose homeostasis.
Summary of Products by Location:
- In Muscles: The primary product usable by the muscle cell is Glucose-6-Phosphate (G6P), which directly feeds into glycolysis. A small amount of free glucose is also produced, which then needs to be phosphorylated to G6P to enter glycolysis.
- In Liver: The primary product released into the bloodstream is Free Glucose. This is generated both directly by the debranching enzyme and, more significantly, by the dephosphorylation of G6P by glucose-6-phosphatase. The liver also produces G6P for its own energy needs.
In essence, glycogenolysis provides either glucose-6-phosphate for immediate energy use within the cell (muscle) or free glucose for systemic distribution (liver).
Glycogenolysis: Regulation
The breakdown of glycogen is under precise control, ensuring that glucose is mobilized only when needed. This regulation involves a combination of hormonal signaling and allosteric control, primarily targeting the key enzyme: Glycogen Phosphorylase.
A central concept is that Glycogen Phosphorylase exists in two forms:
- Glycogen Phosphorylase a (Active Form): The phosphorylated form, highly active.
- Glycogen Phosphorylase b (Less Active Form): The dephosphorylated form, less active and more sensitive to allosteric effectors.
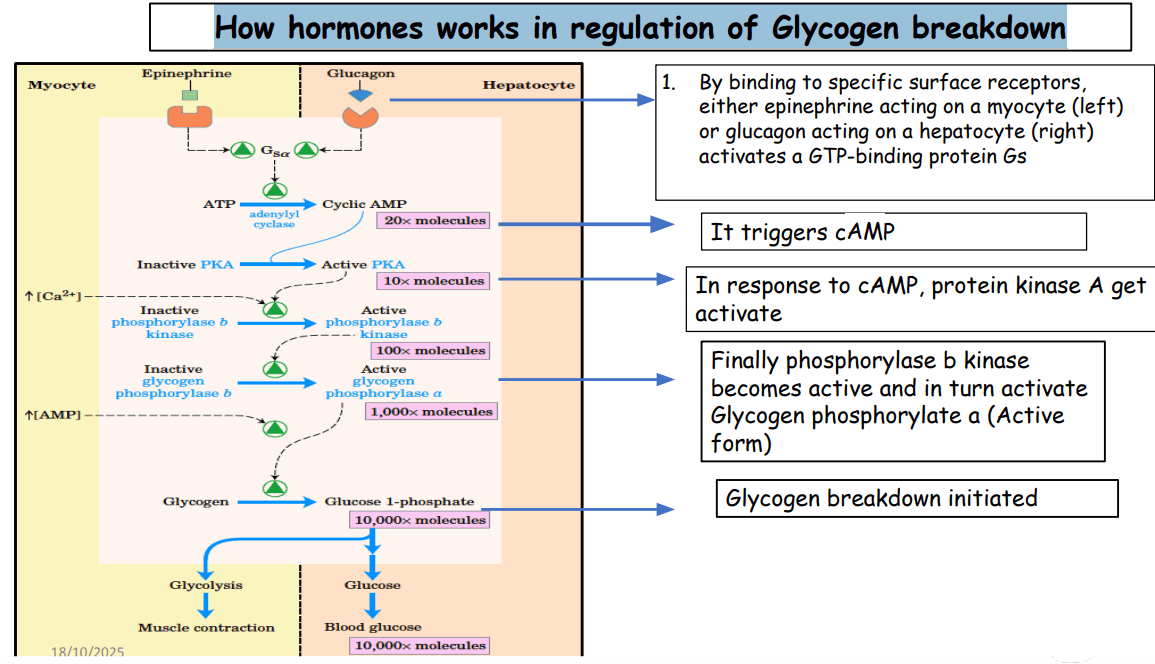
I. Hormonal Control (Covalent Modification via Phosphorylation/Dephosphorylation)
Hormones primarily regulate glycogenolysis by influencing the phosphorylation state of Glycogen Phosphorylase, converting it from the less active 'b' form to the active 'a' form.
Glucagon (Signals Low Blood Glucose)
- Trigger: Released from pancreatic α-cells in response to low blood glucose.
- Action: Primarily targets the liver.
- Mechanism: Glucagon binds to its receptor, activating a pathway that increases cyclic AMP (cAMP). cAMP activates Protein Kinase A (PKA), which in turn activates Phosphorylase Kinase. Finally, Phosphorylase Kinase phosphorylates Glycogen Phosphorylase b, converting it to the active 'a' form.
- Result: Enhanced glycogen breakdown in the liver and release of glucose into the bloodstream.
Epinephrine (Adrenaline - Signals Stress/Energy Demand)
- Trigger: Released from the adrenal medulla in response to stress or intense exercise.
- Action: Targets both the liver and skeletal muscles.
- Mechanism: Similar to glucagon, epinephrine binds to β-adrenergic receptors, increasing cAMP and activating the PKA cascade to convert phosphorylase 'b' to 'a'. In the liver, it can also act via α-adrenergic receptors to increase intracellular Ca²⁺, which also activates Phosphorylase Kinase.
- Result: In the liver, increased glucose release. In muscle, rapid provision of glucose-6-phosphate for immediate ATP production to support contraction.
Insulin (Signals High Blood Glucose)
- Trigger: Released from pancreatic β-cells in response to high blood glucose.
- Action: Promotes glucose storage and inhibits glucose mobilization.
- Mechanism: Insulin primarily counteracts glucagon and epinephrine by activating Protein Phosphatase 1 (PP1). PP1 dephosphorylates Glycogen Phosphorylase 'a', converting it back to the less active 'b' form, effectively turning off glycogenolysis.
II. Allosteric Control (Direct Ligand Binding)
Allosteric regulators bind directly to Glycogen Phosphorylase, rapidly altering its activity to meet immediate cellular needs.
In Skeletal Muscles (Responding to Energy Demand)
- AMP (Adenosine Monophosphate): A potent positive allosteric activator. High AMP signals low energy and activates Glycogen Phosphorylase 'b' even without phosphorylation, providing a rapid "on" switch during intense activity.
- ATP and Glucose-6-Phosphate (G6P): Both are negative allosteric inhibitors. High levels signal sufficient energy, inhibiting Glycogen Phosphorylase 'b' to conserve glycogen.
- Ca²⁺ (Calcium Ions): Released during muscle contraction. Ca²⁺ directly activates Phosphorylase Kinase, leading to the activation of Glycogen Phosphorylase. This directly couples glycogen breakdown to muscle activity.
In Liver (Responding to Blood Glucose Levels)
- Glucose: Acts as a negative allosteric inhibitor of Glycogen Phosphorylase 'a'. When glucose is abundant, it binds to the enzyme, making it a better substrate for dephosphorylation by PP1, effectively turning off glycogenolysis.
Summary of Regulatory Principles:
- Hormonal control (glucagon, epinephrine, insulin) initiates slower, broader responses by modulating the phosphorylation state of Glycogen Phosphorylase.
- Allosteric control (AMP, ATP, Ca²⁺, glucose) provides rapid, fine-tuning adjustments based on the immediate metabolic state of the cell.
Glycogenesis
Glycogenesis is the metabolic pathway responsible for the synthesis of glycogen from glucose. It is the anabolic counterpart to glycogenolysis.
Purpose:
- To store excess glucose when supply is high (e.g., after a meal).
- To maintain blood glucose homeostasis by providing a readily mobilizable glucose reserve in the liver.
- To provide an immediately available energy source for muscle contraction in skeletal muscle.
- To store glucose efficiently without causing osmotic stress, as glycogen is a large polymer.
Location
Glycogenesis occurs primarily in two main tissues, each with a distinct physiological role for the stored glycogen:
Liver:
- Quantity: The liver stores the largest percentage of glycogen by weight (up to 6-8% of its fresh weight).
- Role: Liver glycogen serves as the body's primary glucose reservoir for maintaining blood glucose homeostasis. When blood glucose levels drop, the liver breaks down its glycogen and releases free glucose into the bloodstream to supply other tissues, especially the brain and red blood cells.
Skeletal Muscles:
- Quantity: Skeletal muscles store a lower percentage of glycogen by weight (typically 1-2%), but due to the much larger total mass of muscle, the total amount of glycogen stored often exceeds that in the liver.
- Role: Muscle glycogen serves as a private fuel reserve for the muscle cells themselves. It is primarily used to generate ATP for muscle contraction. Unlike liver glycogen, it cannot be directly released as free glucose into the bloodstream.
Cellular Location: Within both liver and muscle cells, glycogenesis occurs in the cytosol. Glycogen itself is stored in the cytosol as granules, which also contain the enzymes responsible for its synthesis and breakdown.
Key Substrates/Inputs
To synthesize glycogen, the pathway requires specific building blocks and energy sources. The primary substrates are:
Glucose:
- This is the fundamental monosaccharide unit from which glycogen is constructed.
- In the cell, glucose first needs to be phosphorylated to Glucose-6-Phosphate (G6P). This phosphorylation serves several purposes:
- It traps glucose inside the cell, as phosphorylated sugars cannot easily cross the cell membrane.
- It activates glucose for subsequent metabolic reactions.
- The phosphorylation of glucose is catalyzed by:
- Hexokinase in most tissues (including muscle).
- Glucokinase in the liver and pancreatic β-cells.
ATP (Adenosine Triphosphate):
- ATP provides the energy for the initial phosphorylation of glucose to Glucose-6-Phosphate.
- It also provides energy in a later step for the activation of glucose into a UDP-glucose molecule.
UTP (Uridine Triphosphate):
- UTP is crucial for activating glucose, forming UDP-Glucose. This "activated" form of glucose is the direct donor of glucose units for glycogen synthesis.
A Primer (Pre-existing Glycogen or Glycogenin):
- Glycogen synthesis doesn't start from scratch. It requires a pre-existing glycogen molecule (a "primer") to which new glucose units can be added.
- If no glycogen primer is available, a special protein called Glycogenin acts as both an enzyme and a primer. Glycogenin auto-glucosylates itself, forming a short chain of glucose units to which glycogen synthase can then attach further units.
Key Enzymes
The synthesis of glycogen involves several distinct enzymatic steps. We'll highlight the most important ones here.
1. Hexokinase/Glucokinase
- Reaction:
Glucose + ATP → Glucose-6-Phosphate + ADP - Role: Catalyzes the initial phosphorylation of glucose, trapping it inside the cell.
2. Phosphoglucomutase
- Reaction:
Glucose-6-Phosphate ↔ Glucose-1-Phosphate - Role: Reversibly converts G6P to G1P, the precursor for the activated form of glucose.
3. UDP-Glucose Pyrophosphorylase
- Reaction:
Glucose-1-Phosphate + UTP ↔ UDP-Glucose + PPi - Role: Activates glucose by converting G1P into UDP-Glucose, the immediate donor of glucose units. The hydrolysis of the pyrophosphate (PPi) makes this reaction essentially irreversible.
4. Glycogen Synthase
- Reaction:
UDP-Glucose + Glycogenₙ → Glycogenₙ₊₁ + UDP - Role: This is the key regulatory enzyme. It adds glucose units from UDP-glucose to the non-reducing end of a glycogen primer via an
α(1,4)glycosidic bond.
5. Glycogen Branching Enzyme (Amylo-(1,4→1,6)-Transglucosidase)
- Reaction: Transfers a block of
α(1,4)-linked glucose residues to an interior residue via anα(1,6)bond. - Role: Introduces branches into the glycogen molecule.
- Significance: Branching increases solubility and creates numerous non-reducing ends, speeding up both synthesis and degradation.
6. Glycogenin
- Role: Essential for initiating new glycogen molecules. It acts as both a primer and an enzyme, creating a short glucose chain that Glycogen Synthase can then extend.
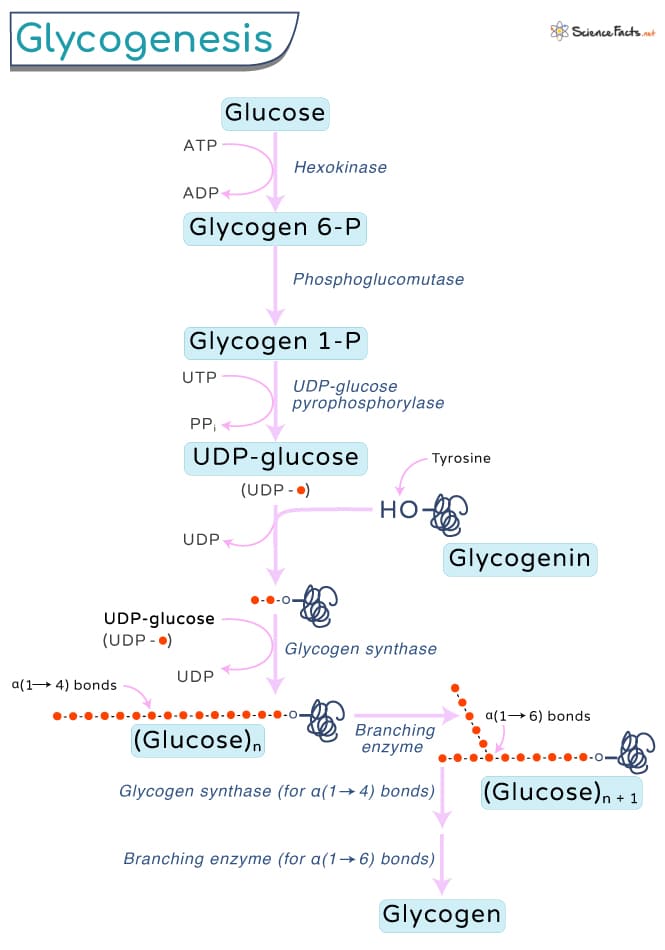
Steps of the Pathway
Glycogenesis can be broken down into a series of coordinated enzymatic reactions:
Step 1: Glucose Phosphorylation
Purpose: To trap and activate glucose inside the cell.
Enzyme: Hexokinase or Glucokinase.
Step 2: Isomerization
Purpose: To convert G6P into the isomer required for activation.
Enzyme: Phosphoglucomutase.
Step 3: Activation to UDP-Glucose
Purpose: To create an "activated" high-energy form of glucose.
Enzyme: UDP-Glucose Pyrophosphorylase.
Step 4: Initiation (Priming)
Purpose: To provide a starting point if no primer exists.
Enzyme: Glycogenin.
Glycogenin auto-glucosylates itself using UDP-Glucose to form a short α(1,4) chain.
Step 5: Elongation
Purpose: To add successive glucose units to the growing chain.
Enzyme: Glycogen Synthase.
Forms new α(1,4) glycosidic bonds.
Step 6: Branching
Purpose: To introduce branches for efficiency.
Enzyme: Glycogen Branching Enzyme.
Transfers a segment of 6-7 glucose units from an α(1,4) chain to an interior position via a new α(1,6) bond.
Products/Outputs
After traversing the steps of the pathway, the primary and most obvious product is:
Glycogen:
- This is the main polymeric carbohydrate storage molecule. It is a large, highly branched polymer of glucose units linked by
α(1,4)andα(1,6)glycosidic bonds. - Stored in the cytosol as granules, particularly abundant in the liver and skeletal muscle.
Beyond the main product, other outputs or byproducts include:
- UDP (Uridine Diphosphate): Released when Glycogen Synthase adds a glucose unit. It is then rephosphorylated back to UTP using ATP (
UDP + ATP ↔ UTP + ADP). - ADP (Adenosine Diphosphate): Released during the initial phosphorylation of glucose and when UDP is rephosphorylated.
- Inorganic Phosphate (Pi): Resulting from the hydrolysis of pyrophosphate (PPi) released during the formation of UDP-glucose.
Regulation
The synthesis of glycogen is a tightly regulated process. The most important regulatory enzyme is Glycogen Synthase.
A. Hormonal Regulation (via Covalent Modification)
Hormones signal the body's metabolic state, leading to the phosphorylation or dephosphorylation of glycogen synthase to alter its activity.
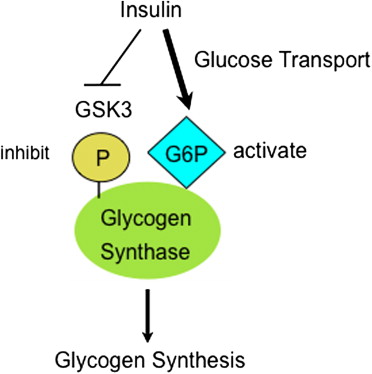
Insulin (High Blood Glucose)
Effect: Promotes glycogenesis.
Mechanism: Insulin activates Protein Phosphatase 1 (PP1), which dephosphorylates Glycogen Synthase, converting it to its active 'a' form (GSa).
Glucagon (Low Blood Glucose)
Effect: Inhibits glycogenesis (in liver).
Mechanism: Glucagon activates Protein Kinase A (PKA), which phosphorylates Glycogen Synthase, converting it to its inactive 'b' form (GSb).
Epinephrine (Fight-or-Flight)
Effect: Inhibits glycogenesis (in liver & muscle).
Mechanism: Similar to glucagon, epinephrine activates PKA, which phosphorylates and inactivates Glycogen Synthase (GSb).
B. Allosteric Regulation
Allosteric regulators directly bind to enzymes in response to the cellular energy state.
- Glucose-6-Phosphate (G6P): Allosterically activates Glycogen Synthase (specifically the 'b' form). When G6P levels are high, it signals a surplus of glucose ready for storage, promoting glycogen synthesis even before hormonal signals fully kick in.
C. Other Factors
- Calcium (Ca²⁺) and AMP (Muscle Specific): During muscle contraction, Ca²⁺ is released and AMP levels rise. These signals strongly activate glycogen breakdown (glycogenolysis), which generally suppresses synthesis.
- Substrate Availability: The availability of UDP-Glucose also influences the rate of synthesis.
Summary of Regulation:
- High Glucose / Fed State: Insulin dominates. It leads to dephosphorylation of Glycogen Synthase, making it active (GSa). Result: Glycogen Synthesis.
- Low Glucose / Fasted State: Glucagon dominates (liver). It leads to phosphorylation of Glycogen Synthase, making it inactive (GSb). Result: Glycogen Breakdown.
- Stress / Exercise: Epinephrine dominates. It leads to phosphorylation and inactivation of Glycogen Synthase. Result: Glycogen Breakdown.
Biochemistry: Glycogenesis & Glycogenolysis Exam
Test your knowledge with these 40 questions.
Glycogen Metabolism Exam
Question 1/40
Exam Complete!
Here are your results, .
Your Score
38/40
95%