Gluconeogenesis: New Glucose
GLUCONEOGENESIS
The term "gluconeogenesis" literally means "new formation of glucose" (from Greek: glykys = sweet, neos = new, genesis = origin). It is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as lactate, glycerol, and certain amino acids.
Primary Purpose:
The purpose of gluconeogenesis is to maintain blood glucose homeostasis, especially during periods when carbohydrate intake is insufficient (e.g., fasting, starvation, prolonged exercise).
Why is this critical? The brain and red blood cells rely almost exclusively on glucose for their energy needs. Without gluconeogenesis, blood glucose levels would drop dangerously low (hypoglycemia) once glycogen stores are depleted, leading to severe physiological consequences.
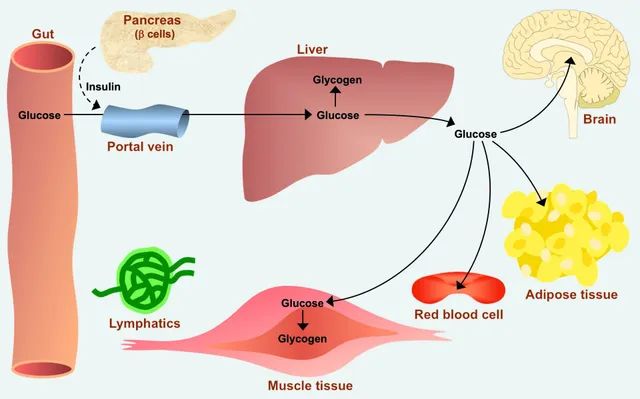
Primary Tissues/Organs:
Gluconeogenesis primarily occurs in two organs:
- Liver (Hepatic Gluconeogenesis): This is the major site of gluconeogenesis. The liver can synthesize glucose and release it into the bloodstream for use by other tissues. Approximately 90% of all gluconeogenesis occurs in the liver.
- Kidney (Renal Gluconeogenesis): The kidneys also play a significant role, especially during prolonged fasting. The kidney can contribute up to 10% of glucose production during an overnight fast, and up to 40% during prolonged starvation.
Key Precursors:
Gluconeogenesis utilizes various non-carbohydrate molecules as starting materials. These precursors are ultimately converted into oxaloacetate, which then proceeds through the pathway. The three main classes are:
1. Lactate:
- Origin: Produced by anaerobic glycolysis in actively contracting skeletal muscle and in red blood cells.
- Conversion: Lactate is transported to the liver, where it is converted back to pyruvate by lactate dehydrogenase. This cycle (muscle lactate to liver glucose) is known as the Cori Cycle.
2. Amino Acids (Glucogenic Amino Acids):
- Origin: Derived primarily from the breakdown of muscle protein, especially during fasting.
- Conversion: The carbon skeletons of many amino acids can be converted into pyruvate or TCA cycle intermediates (e.g.,
α-ketoglutarate, succinyl-CoA). Alanine is particularly important, forming the Glucose-Alanine Cycle. - Note: Fatty acids cannot be directly converted to glucose in animals because the conversion of acetyl-CoA (from fatty acid breakdown) to pyruvate or oxaloacetate is not possible.
3. Glycerol:
- Origin: Released during the hydrolysis of triglycerides (fats) in adipose tissue.
- Conversion: Glycerol is transported to the liver, where it is phosphorylated and then oxidized to dihydroxyacetone phosphate (DHAP). DHAP is an intermediate in both glycolysis and gluconeogenesis, readily entering the pathway.
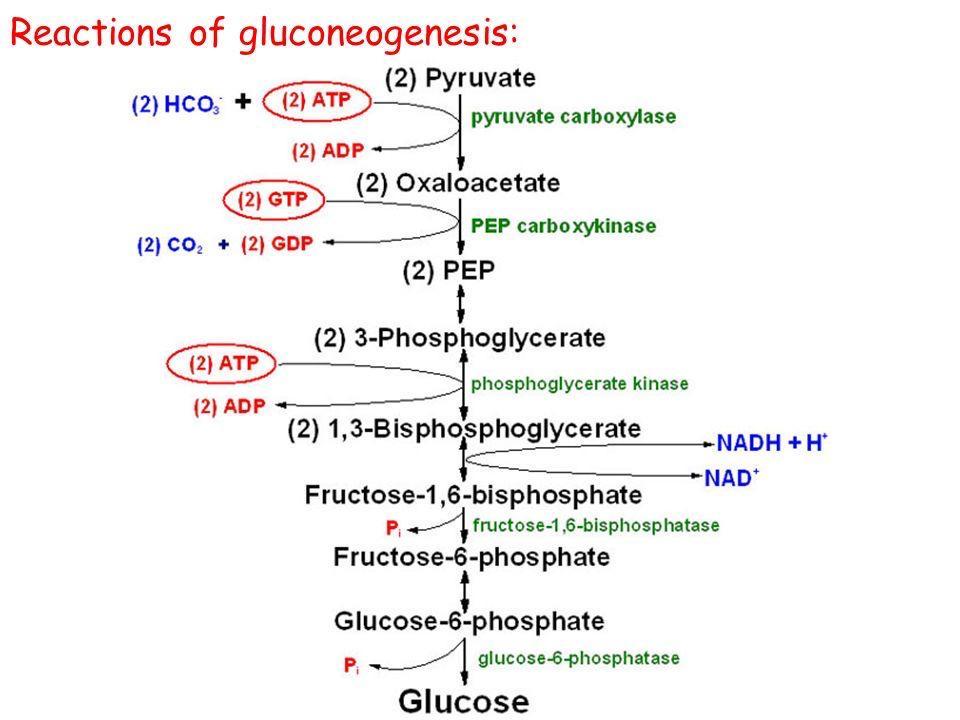
Major Steps and Bypassing Irreversible Glycolysis Reactions
Gluconeogenesis is NOT simply the reversal of glycolysis. While it shares many reversible steps, there are three highly exergonic (irreversible) steps in glycolysis that must be bypassed by different enzymes in gluconeogenesis. These bypasses are crucial for the pathway to be thermodynamically favorable and for regulatory control.
Overview of the Pathway (from Pyruvate to Glucose)
The overall process can be thought of as reversing glycolysis, but with four unique "bypass" reactions:
- Bypass 1: Pyruvate → Phosphoenolpyruvate (PEP)
- Bypass 2: Fructose-1,6-bisphosphate → Fructose-6-phosphate
- Bypass 3: Glucose-6-phosphate → Glucose
Detailed Steps & Key Enzymes
Let's start from pyruvate, a common entry point for lactate and some amino acids.
1. Pyruvate to Phosphoenolpyruvate (PEP) - The First Bypass
This bypass replaces the highly irreversible pyruvate kinase step of glycolysis. It requires two enzymes and crosses the mitochondrial membrane.
Step 1a: Pyruvate to Oxaloacetate (in Mitochondria)
- Enzyme: Pyruvate Carboxylase (PC)
- Reaction:
Pyruvate + CO₂ + ATP → Oxaloacetate + ADP + Pi - Cofactor: Biotin (carries CO₂)
- Key Point: This enzyme is in the mitochondrial matrix and is allosterically activated by acetyl-CoA. High acetyl-CoA signals that pyruvate should be directed towards glucose synthesis.
Step 1b: Oxaloacetate to PEP (Mitochondria and/or Cytosol)
Oxaloacetate cannot directly cross the mitochondrial membrane. It must first be converted via one of two options, often involving a malate shuttle, to generate cytosolic NADH which is needed later.
- Enzyme: PEP Carboxykinase (PEPCK)
- Reaction:
Oxaloacetate + GTP → PEP + GDP + CO₂
2. PEP to Fructose-1,6-bisphosphate
From PEP, the pathway essentially reverses the reversible steps of glycolysis using the same enzymes, but in the reverse direction:
- PEP → 2-Phosphoglycerate → 3-Phosphoglycerate (via Enolase, Phosphoglycerate mutase)
- 3-Phosphoglycerate → 1,3-Bisphosphoglycerate (via Phosphoglycerate kinase, consuming ATP)
- 1,3-Bisphosphoglycerate → Glyceraldehyde-3-phosphate (via Glyceraldehyde-3-phosphate dehydrogenase, consuming NADH)
- Glyceraldehyde-3-phosphate ↔ Dihydroxyacetone phosphate (DHAP) (via Triose phosphate isomerase). DHAP from glycerol enters here.
- Glyceraldehyde-3-phosphate + DHAP → Fructose-1,6-bisphosphate (via Aldolase)
3. Fructose-1,6-bisphosphate to Fructose-6-phosphate - The Second Bypass
This bypass replaces the irreversible phosphofructokinase-1 (PFK-1) step of glycolysis.
- Enzyme: Fructose-1,6-bisphosphatase (FBPase-1)
- Reaction:
Fructose-1,6-bisphosphate + H₂O → Fructose-6-phosphate + Pi - Key Point: This is a hydrolysis reaction, releasing inorganic phosphate (Pi). It is a critical, reciprocally regulated point with PFK-1.
4. Fructose-6-phosphate to Glucose-6-phosphate
- Enzyme: Phosphohexose isomerase (reversible, same as glycolysis)
- Reaction:
Fructose-6-phosphate ↔ Glucose-6-phosphate
5. Glucose-6-phosphate to Free Glucose - The Third Bypass
This bypass replaces the irreversible hexokinase/glucokinase step of glycolysis.
- Enzyme: Glucose-6-phosphatase
- Reaction:
Glucose-6-phosphate + H₂O → Glucose + Pi - Key Point: This enzyme is found primarily in the liver and kidney and is located in the endoplasmic reticulum membrane. It allows free glucose to be released into the bloodstream. Muscle cells lack this enzyme.
Summary of the Bypasses and Key Enzymes:
| Glycolysis Irreversible Step (Enzyme) | Gluconeogenesis Bypass Enzyme(s) | Location |
|---|---|---|
Glucose → G6P (Hexokinase/Glucokinase) |
Glucose-6-phosphatase | ER lumen (liver, kidney) |
F6P → FBP (PFK-1) |
Fructose-1,6-bisphosphatase (FBPase-1) | Cytosol |
PEP → Pyruvate (Pyruvate Kinase) |
1. Pyruvate Carboxylase 2. PEP Carboxykinase (PEPCK) |
Mitochondria & Cytosol |
Energy Requirements
Synthesizing glucose from non-carbohydrate precursors is an energy-intensive, anabolic process. Let's calculate the ATP and GTP expenditure required to synthesize one molecule of glucose from two molecules of pyruvate.
Here's a breakdown of the energy-consuming steps:
-
Pyruvate to Oxaloacetate (x2):
- Catalyzed by Pyruvate Carboxylase, this step consumes 1 ATP per pyruvate.
- Total cost: 2 ATP
-
Oxaloacetate to Phosphoenolpyruvate (PEP) (x2):
- Catalyzed by PEP Carboxykinase, this step consumes 1 GTP per oxaloacetate.
- Total cost: 2 GTP
-
3-Phosphoglycerate to 1,3-Bisphosphoglycerate (x2):
- Catalyzed by Phosphoglycerate Kinase, this step consumes 1 ATP per 3-phosphoglycerate.
- Total cost: 2 ATP
Total Energy Cost for Synthesizing one Glucose from two Pyruvates:
Important Considerations:
- NADH Requirement: In addition to ATP and GTP, the pathway consumes 2 NADH during the conversion of 1,3-bisphosphoglycerate to glyceraldehyde-3-phosphate.
- Energy Balance and Futile Cycles: This significant energy investment highlights why gluconeogenesis and glycolysis must be reciprocally regulated. If both were active simultaneously, it would result in a "futile cycle," simply burning ATP and GTP to generate heat.
Reciprocal Regulation with Glycolysis
To prevent a wasteful "futile cycle," glycolysis and gluconeogenesis are reciprocally regulated. Conditions that activate one pathway typically inhibit the other. This occurs at the three irreversible steps.
Pyruvate Kinase ↔ Pyruvate Carboxylase / PEPCK
- High ATP & Alanine: Inhibit Pyruvate Kinase (Glycolysis).
- High Acetyl-CoA: Activates Pyruvate Carboxylase (Gluconeogenesis). This is a key signal from fatty acid breakdown, diverting pyruvate to glucose synthesis.
PFK-1 ↔ FBPase-1 (The Main Control Point)
- High ATP & Citrate: Inhibit PFK-1 (Glycolysis), signaling high energy.
- High AMP: Activates PFK-1 (Glycolysis) and inhibits FBPase-1 (Gluconeogenesis), signaling low energy.
- Fructose-2,6-bisphosphate (F2,6BP): This is the most potent regulator.
- High F2,6BP: Strongly activates PFK-1 (Glycolysis) and inhibits FBPase-1 (Gluconeogenesis).
- Low F2,6BP: Relieves inhibition of FBPase-1, promoting Gluconeogenesis.
- How is F2,6BP regulated? Its levels are controlled by a bifunctional enzyme (PFK-2/FBPase-2), which is in turn regulated by insulin (increases F2,6BP) and glucagon (decreases F2,6BP).
Hexokinase/Glucokinase ↔ Glucose-6-Phosphatase
- High Glucose-6-Phosphate (G6P): Inhibits Hexokinase (Glycolysis).
- Transcriptional Control: The gene expression of Glucose-6-Phosphatase is significantly upregulated by glucagon and inhibited by insulin, a long-term adaptation to fasting.
Summary of Reciprocal Regulation
| Regulatory Molecule | Glycolysis (Effect) | Gluconeogenesis (Effect) | Physiological Context |
|---|---|---|---|
| High ATP | ↓ (Inhibits) | ↑ (Activates) | High energy state |
| High AMP | ↑ (Activates) | ↓ (Inhibits) | Low energy state |
| High Citrate | ↓ (Inhibits) | -- | Abundant TCA intermediates |
| High Acetyl-CoA | ↓ (Inhibits) | ↑ (Activates) | Fatty acid oxidation |
| High F2,6BP | ↑ (Activates) | ↓ (Inhibits) | High glucose (Insulin) |
| Low F2,6BP | ↓ (Inhibits) | ↑ (Activates) | Low glucose (Glucagon) |
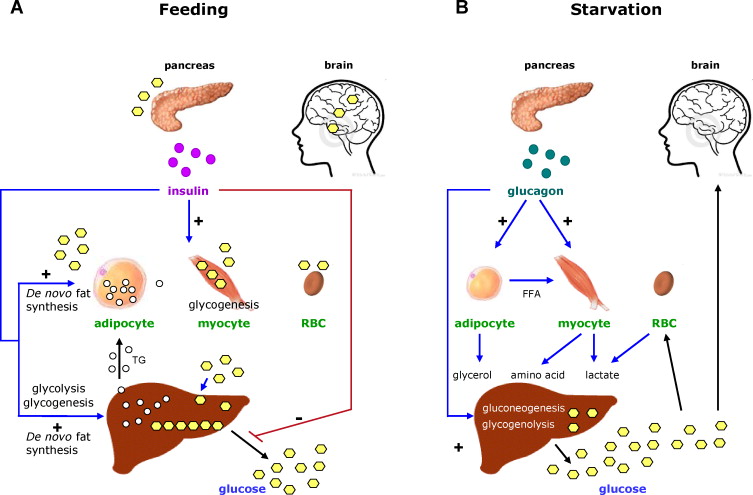
Hormonal Control
The activity of gluconeogenesis is tightly regulated by hormones that respond to changes in blood glucose levels and overall energy status. The primary hormones involved are glucagon, insulin, and cortisol.
1. Glucagon (The "Low Blood Glucose" Hormone)
- Released from: Alpha cells of the pancreas.
- Stimulus: Low blood glucose (hypoglycemia).
- Target Tissue: Primarily the liver (muscle cells lack glucagon receptors).
- Mechanism: Glucagon binds to its receptor, increasing intracellular cAMP, which activates Protein Kinase A (PKA). PKA then phosphorylates the bifunctional PFK-2/FBPase-2 enzyme, activating its FBPase-2 domain. This decreases the concentration of Fructose-2,6-bisphosphate (F2,6BP).
- Overall Effect: Lower F2,6BP levels inhibit glycolysis (PFK-1) and activate gluconeogenesis (FBPase-1). PKA also promotes the gene expression of gluconeogenic enzymes. This stimulates gluconeogenesis to raise blood glucose.
2. Insulin (The "High Blood Glucose" Hormone)
- Released from: Beta cells of the pancreas.
- Stimulus: High blood glucose (hyperglycemia).
- Target Tissues: Liver, muscle, and adipose tissue.
- Mechanism: Insulin activates Protein Phosphatase 1 (PP1). PP1 dephosphorylates the bifunctional PFK-2/FBPase-2 enzyme, activating its PFK-2 domain. This increases the concentration of Fructose-2,6-bisphosphate (F2,6BP).
- Overall Effect: Higher F2,6BP levels activate glycolysis (PFK-1) and inhibit gluconeogenesis (FBPase-1). Insulin also suppresses the gene expression of gluconeogenic enzymes. This inhibits gluconeogenesis to lower blood glucose.
3. Cortisol (A "Stress" Hormone)
- Released from: Adrenal cortex.
- Stimulus: Stress, prolonged fasting.
- Mechanism: Cortisol acts primarily by regulating gene expression over a longer time frame. It increases the transcription of genes for key gluconeogenic enzymes (Pyruvate Carboxylase, PEPCK, FBPase-1, Glucose-6-Phosphatase).
- Overall Effect: Cortisol enhances gluconeogenesis by providing both enzymes and substrates (by promoting muscle protein breakdown), contributing to maintaining blood glucose during prolonged stress or fasting.
Summary of Hormonal Effects on Gluconeogenesis:
| Hormone | Physiological Context | Effect on Gluconeogenesis | Primary Mechanism |
|---|---|---|---|
| Glucagon | Low blood glucose (fasting) | Stimulates | Activates PKA → decreases F2,6BP → activates FBPase-1; increases gene expression. |
| Insulin | High blood glucose (fed state) | Inhibits | Activates PP1 → increases F2,6BP → inhibits FBPase-1; decreases gene expression. |
| Cortisol | Stress, prolonged fasting | Stimulates | Increases gene expression of gluconeogenic enzymes; mobilizes amino acid precursors. |
A. Connection to Physiological States
Gluconeogenesis is vital for maintaining metabolic homeostasis under various conditions.
Fasting (Short-Term)
During an overnight fast (12-24 hours), gluconeogenesis supplements glycogenolysis. As glycogen stores deplete, it becomes the primary source of glucose.
Precursors: Lactate, alanine, and glycerol.
Hormones: High glucagon, low insulin.
Starvation (Long-Term)
After 24 hours, gluconeogenesis is essential for survival, providing all glucose for the brain and RBCs. To spare muscle protein, the body shifts to using fatty acids and ketone bodies as primary fuel.
Hormones: High glucagon, low insulin, elevated cortisol.
The kidneys significantly increase their contribution (up to 40%).
Prolonged Exercise
During endurance exercise, gluconeogenesis helps maintain blood glucose. The liver efficiently recycles lactate (Cori Cycle) and alanine (Glucose-Alanine Cycle) produced by muscles.
Hormones: Increased glucagon and epinephrine.
High-Protein Diet
If carbohydrate intake is very low, gluconeogenesis ensures a sufficient supply of glucose by using amino acids derived from dietary protein as the primary precursors.
B. Clinical Relevance
Dysregulation of gluconeogenesis is central to several metabolic disorders.
Diabetes Mellitus
A hallmark of diabetes is overproduction of glucose by the liver due to unrestrained gluconeogenesis, contributing significantly to hyperglycemia.
- Type 1: Absence of insulin means glucagon's effects are unopposed.
- Type 2: The liver becomes resistant to insulin's signal to suppress gluconeogenesis.
Therapeutic Target: Metformin, a common diabetes drug, works primarily by inhibiting hepatic gluconeogenesis.
Alcohol Consumption
Heavy alcohol consumption can lead to hypoglycemia by inhibiting gluconeogenesis, especially in a fasted state.
Mechanism: Alcohol metabolism generates a large amount of NADH. This high NADH/NAD⁺ ratio shifts key reactions away from gluconeogenesis precursors (converts pyruvate to lactate and oxaloacetate to malate), starving the pathway.
Genetic Disorders
- Fructose-1,6-bisphosphatase Deficiency: A rare disorder where the FBPase-1 enzyme is deficient. Patients cannot synthesize glucose efficiently, leading to severe hypoglycemia and lactic acidosis, especially during fasting.
- Von Gierke's Disease (GSD Type I): A deficiency in Glucose-6-phosphatase, the final enzyme of both gluconeogenesis and glycogenolysis. This leads to severe fasting hypoglycemia, lactic acidosis, and an enlarged liver (hepatomegaly) because glucose cannot be released.
Biochemistry: Gluconeogenesis Exam
Test your knowledge with these 30 questions.
Gluconeogenesis Exam
Question 1/30
Exam Complete!
Here are your results, .
Your Score
28/30
93%