Fatty Acid : Metabolism
Fatty Acid Metabolism
Fatty acids are fundamental molecules in biology, playing roles in energy, structure, and signaling. Their metabolism is highly regulated and central to energy homeostasis in the body. For more details on Fatty Acids, Click Here.
Briefly, What are Fatty Acids?
Fatty acids are long hydrocarbon chains with a carboxyl group (-COOH) at one end. This makes them amphipathic molecules, meaning they have both hydrophobic (the hydrocarbon chain) and hydrophilic (the carboxyl group) regions. They are found esterified to glycerol in triacylglycerols (TAGs) or as components of phospholipids and sphingolipids. In their free form, they are called free fatty acids (FFAs).
Classification of Fatty Acids
A. Based on Saturation:
- Saturated Fatty Acids (SFAs): Contain no carbon-carbon double bonds. Examples: Palmitic acid (16:0), Stearic acid (18:0). Tend to be solid at room temperature.
- Unsaturated Fatty Acids (UFAs): Contain one or more carbon-carbon double bonds.
- Monounsaturated (MUFAs): Have one double bond (e.g., Oleic acid).
- Polyunsaturated (PUFAs): Have two or more double bonds (e.g., Linoleic acid).
B. Based on Chain Length:
- Short-Chain (SCFAs): 2 to 4 carbons.
- Medium-Chain (MCFAs): 6 to 12 carbons.
- Long-Chain (LCFAs): 14 to 20 carbons (most common).
- Very Long-Chain (VLCFAs): >20 carbons.
C. Based on Essentiality:
- Non-Essential Fatty Acids: Can be synthesized by the body.
- Essential Fatty Acids (EFAs): Cannot be synthesized and must be obtained from the diet.
- Linoleic Acid (Omega-6): Precursor to arachidonic acid.
- α-Linolenic Acid (Omega-3): Precursor to EPA and DHA.
Major Physiological Roles of Fatty Acids
Fatty acids are multifaceted molecules critical for life.
Energy Storage
Stored as triacylglycerols (TAGs), they are the body's most concentrated and efficient form of long-term energy storage, yielding more ATP per gram than carbohydrates.
Structural Components
They are integral components of phospholipids and sphingolipids, which form the fundamental structure of all biological membranes.
Signaling & Precursors
Essential fatty acids are precursors to powerful local signaling molecules called eicosanoids (prostaglandins, thromboxanes, leukotrienes) involved in inflammation, pain, and blood clotting.
Insulation & Absorption
Adipose tissue provides thermal insulation and protection for organs. Dietary fats are also necessary for the absorption of fat-soluble vitamins (A, D, E, K).
Primary Metabolic States: Fed vs. Fasted
The body meticulously regulates fatty acid metabolism based on energy availability.
Fed State (High Energy / Insulin Dominant)
After a meal, excess carbohydrates and proteins are converted into fatty acids (Lipogenesis) and stored as TAGs in adipose tissue. The goal is to store energy.
Fasted State (Low Energy / Glucagon Dominant)
When nutrient intake is low, stored TAGs are broken down, releasing fatty acids. These are then broken down for energy (Beta-Oxidation). The goal is to release stored energy.
Major Pathways Involved in Fatty Acid Metabolism
- Fatty Acid Synthesis (Lipogenesis): The process of building fatty acids from Acetyl-CoA. Occurs primarily in the cytosol.
- Fatty Acid Oxidation (Beta-Oxidation): The pathway that breaks down fatty acids into Acetyl-CoA to generate energy. Occurs primarily in the mitochondrial matrix.
- Triacylglycerol (TAG) Synthesis and Degradation: The processes of storing (esterification) and mobilizing (lipolysis) fatty acids.
- Ketone Body Metabolism:
- Ketogenesis: The liver converts excess Acetyl-CoA into ketone bodies during prolonged fasting.
- Ketolysis: Other tissues use ketone bodies as an alternative fuel source.
Fatty Acid Mobilization and Transport
When energy is needed, stored triacylglycerols (TAGs) in adipose tissue must be broken down, and the resulting fatty acids transported to other tissues for oxidation.
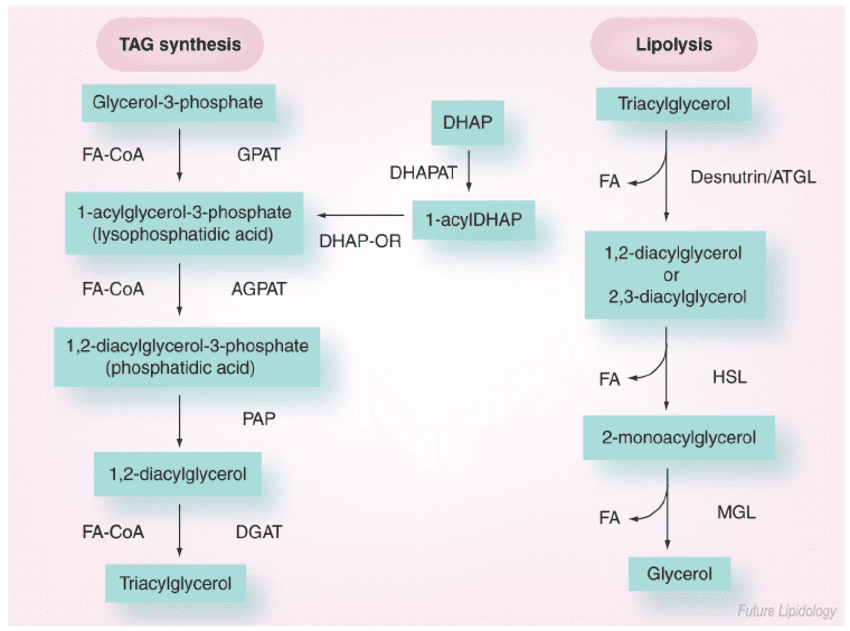
1. Triacylglycerol (TAG) Mobilization (Lipolysis)
Lipolysis is the process of breaking down stored TAGs into fatty acids and glycerol, occurring in adipocytes.
- Stimuli: Hormones like epinephrine, norepinephrine, and glucagon signal a low-energy state and activate lipolysis. Insulin inhibits it.
- Key Players (Lipases):
- Hormone-Sensitive Lipase (HSL): The rate-limiting enzyme, activated by phosphorylation via a PKA-dependent pathway.
- Adipose Triglyceride Lipase (ATGL): Initiates the first step, converting TAGs to DAGs.
- Monoacylglycerol Lipase (MAGL): Catalyzes the final step.
- Products of Lipolysis: Free Fatty Acids (FFAs) and Glycerol are released into the bloodstream.
- Fate of Glycerol: Travels to the liver, where it can enter glycolysis or gluconeogenesis. Adipocytes lack the enzyme (glycerol kinase) to re-utilize it.
2. Transport of Free Fatty Acids (FFAs) in Blood
Long-chain fatty acids are hydrophobic and require a carrier in the blood.
- Carrier Protein: Albumin, the most abundant plasma protein, serves as the primary carrier for FFAs.
- Mechanism: FFAs bind non-covalently to hydrophobic pockets on the albumin molecule.
- Delivery to Tissues: Fatty acid-albumin complexes deliver FFAs to tissues like muscle and heart, where they are taken up by specific fatty acid transporters.
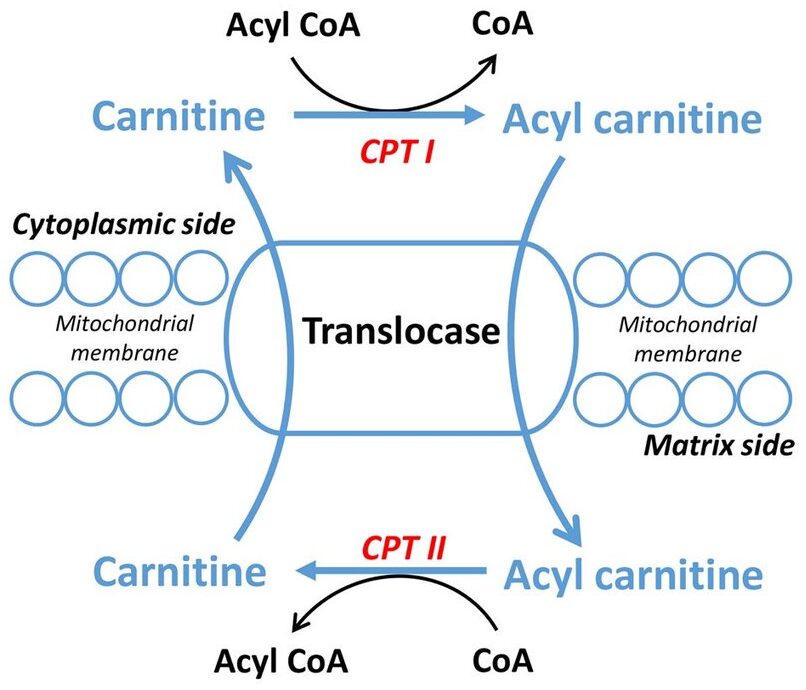
3. Transport into Mitochondria (The Carnitine Shuttle)
Long-chain fatty acids (LCFAs) cannot directly cross the inner mitochondrial membrane. They require the Carnitine Shuttle to enter the mitochondrial matrix for beta-oxidation.
Steps of the Shuttle:
- Activation (Cytosol): The FFA is first activated to a fatty acyl-CoA by Fatty Acyl-CoA Synthetase, consuming 2 ATP equivalents.
- Transfer to Carnitine (Outer Membrane): The fatty acyl group is transferred from CoA to carnitine by Carnitine Palmitoyltransferase I (CPT-I), forming fatty acylcarnitine. CPT-I is the rate-limiting step and is inhibited by malonyl-CoA.
- Translocation (Inner Membrane): Carnitine-Acylcarnitine Translocase (CACT) transports fatty acylcarnitine into the matrix while simultaneously transporting a free carnitine out.
- Transfer Back to CoA (Matrix): Inside the matrix, Carnitine Palmitoyltransferase II (CPT-II) transfers the fatty acyl group back to a mitochondrial CoA, regenerating fatty acyl-CoA (now ready for beta-oxidation) and freeing carnitine for reuse.
Now, with the fatty acyl-CoA ready in the mitochondrial matrix, we can move on to the actual breakdown process: Fatty Acid Oxidation (Beta-Oxidation).
Fatty Acid Oxidation (Beta-Oxidation)
Once long-chain fatty acids (as fatty acyl-CoA) have successfully entered the mitochondrial matrix via the carnitine shuttle, they are ready for a cyclic process called β-oxidation. This pathway systematically cleaves two-carbon units from the carboxyl end of the fatty acyl-CoA, generating acetyl-CoA, NADH, and FADH₂, which then feed into the citric acid cycle and oxidative phosphorylation for ATP production.
- Primary Location: Mitochondrial matrix.
- Purpose: To generate energy (ATP) from stored fatty acids.
The Sequential Steps of β-Oxidation for Saturated Fatty Acyl-CoAs
Beta-oxidation is a four-step cyclic process. Each cycle shortens the fatty acyl-CoA by two carbons and produces one molecule of Acetyl-CoA, one NADH, and one FADH₂.
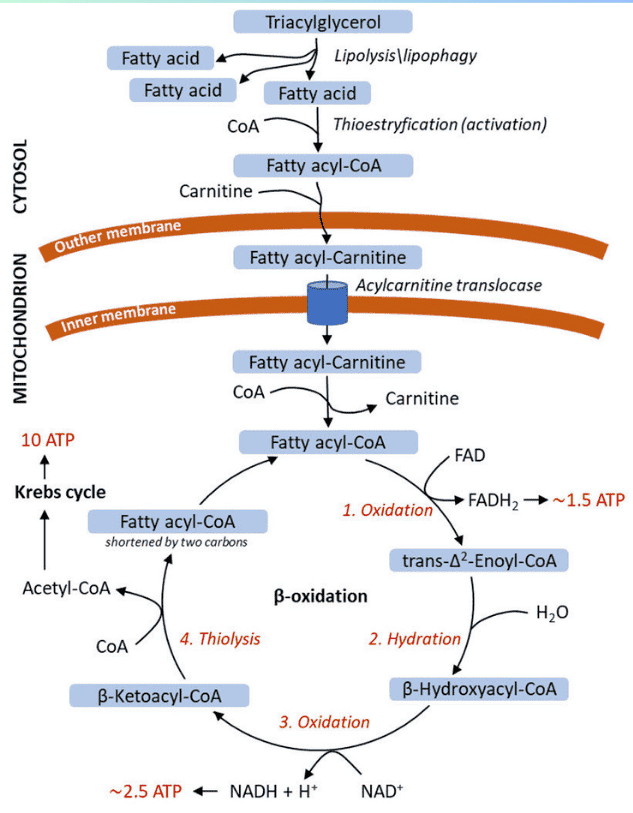
The Four Steps of One Cycle:
-
Oxidation (by FAD):
- Enzyme: Acyl-CoA Dehydrogenase (specific for chain length, e.g., VLCAD, LCAD, MCAD, SCAD).
- Reaction: Introduces a trans double bond between the α (C-2) and β (C-3) carbons of the fatty acyl-CoA, producing a trans-Δ²-enoyl-CoA.
- Product: FADH₂ (reduced flavin adenine dinucleotide). This FADH₂ then donates its electrons to Coenzyme Q in the electron transport chain, yielding ~1.5 ATP.
-
Hydration:
- Enzyme: Enoyl-CoA Hydratase (also known as Crotonase).
- Reaction: Adds water across the double bond of the trans-Δ²-enoyl-CoA, forming a hydroxyl group on the β-carbon. This produces L-β-hydroxyacyl-CoA.
-
Oxidation (by NAD⁺):
- Enzyme: β-hydroxyacyl-CoA Dehydrogenase.
- Reaction: Oxidizes the hydroxyl group on the β-carbon to a ketone group, producing β-ketoacyl-CoA.
- Product: NADH (reduced nicotinamide adenine dinucleotide). This NADH then donates its electrons to Complex I of the electron transport chain, yielding ~2.5 ATP.
-
Thiolytic Cleavage (Thiolysis):
- Enzyme: β-ketoacyl-CoA Thiolase (also known as Acyl-CoA Acetyltransferase).
- Reaction: Cleaves the bond between the α and β carbons. A molecule of Coenzyme A (CoA-SH) attacks the β-keto carbon, releasing one molecule of Acetyl-CoA and a new fatty acyl-CoA that is two carbons shorter than the original.
- Products: Acetyl-CoA (enters the Citric Acid Cycle) and a shortened fatty acyl-CoA (which re-enters the β-oxidation cycle).
Summary of One Cycle of β-Oxidation:
Input: Fatty Acyl-CoA (n carbons) → Output: 1 Acetyl-CoA + 1 FADH₂ + 1 NADH + Fatty Acyl-CoA (n-2 carbons)
Calculating the Net ATP Yield from Palmitate (16:0)
- Number of carbons: 16
- Number of Acetyl-CoA units produced: 16 / 2 = 8 Acetyl-CoA.
- Number of β-oxidation cycles needed: 8 - 1 = 7 cycles.
- ATP Yield Calculation:
- From β-oxidation cycles:
- 7 cycles × 1 FADH₂/cycle = 7 FADH₂
- 7 FADH₂ × 1.5 ATP/FADH₂ = 10.5 ATP
- 7 cycles × 1 NADH/cycle = 7 NADH
- 7 NADH × 2.5 ATP/NADH = 17.5 ATP
- Total from cycles = 10.5 + 17.5 = 28 ATP
- From Acetyl-CoA entering the Citric Acid Cycle (TCA Cycle):
- 8 Acetyl-CoA × (1 FADH₂ + 3 NADH + 1 GTP)/Acetyl-CoA
- 8 FADH₂ × 1.5 ATP/FADH₂ = 12 ATP
- 8 NADH × 2.5 ATP/NADH = 20 ATP
- 8 GTP × 1 ATP/GTP = 8 ATP
- Total from Acetyl-CoA = 12 + 20 + 8 = 40 ATP
- Initial Activation Cost:
- Activating the fatty acid consumes 2 ATP equivalents.
- Cost = -2 ATP
- From β-oxidation cycles:
- Net ATP Yield: (28 from cycles) + (40 from Acetyl-CoA) - 2 (activation) = 106 ATP.
Modifications for Unsaturated and Odd-Chain Fatty Acids
A. Unsaturated Fatty Acids:
- Problem: The presence of double bonds interferes with the standard pathway.
- Solutions:
- Enoyl-CoA Isomerase: For cis double bonds, this enzyme converts them to the trans form, bypassing the FADH₂-producing step in that cycle.
- 2,4-Dienoyl-CoA Reductase: For polyunsaturated fatty acids, this reductase (requiring NADPH) helps handle conjugated double bonds.
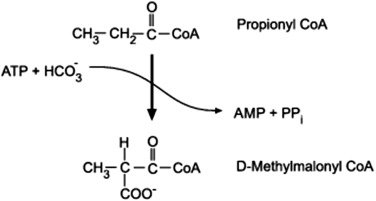
B. Odd-Chain Fatty Acids:
- Problem: The final cycle yields one Propionyl-CoA (3 carbons), which cannot enter the TCA cycle directly.
- Solution (Propionyl-CoA Pathway): Propionyl-CoA is converted to Succinyl-CoA (a TCA cycle intermediate) in a multi-step process requiring Biotin and Vitamin B12.
- Significance: This makes odd-chain fatty acids the only fatty acids that can yield a net glucose precursor.
Other Forms of Fatty Acid Oxidation
A. α-Oxidation:
- Location: Peroxisomes and Endoplasmic Reticulum.
- Purpose: Degrades fatty acids with a methyl group on the β-carbon (e.g., phytanic acid).
- Process: Removes one carbon at a time from the carboxyl end.
- Clinical Significance: A defect causes Refsum disease, leading to neurological damage.
B. ω-Oxidation:
- Location: Endoplasmic Reticulum of the liver and kidneys.
- Purpose: A minor pathway that becomes more important when β-oxidation is defective.
- Process: Oxidizes the methyl (ω) carbon at the opposite end of the chain, creating a dicarboxylic acid that can then undergo β-oxidation from both ends.
- Products: Succinate (4 carbons) and Adipate (6 carbons).
Ketone Body Metabolism (Ketogenesis and Ketolysis)
Under certain physiological conditions, particularly prolonged fasting, starvation, or uncontrolled diabetes, the liver produces significant amounts of ketone bodies from Acetyl-CoA. These ketone bodies serve as an alternative fuel source for extrahepatic (outside the liver) tissues, especially the brain, which cannot directly use fatty acids for energy.
Conditions That Lead to Ketogenesis
Ketogenesis is stimulated when:
- Low Glucose Availability: This is the primary driver. When glucose is scarce, the body turns to fat as its main energy source.
- High Rate of Fatty Acid Oxidation: Increased breakdown of fatty acids in the liver leads to an abundance of Acetyl-CoA.
- Low Oxaloacetate (OAA) Levels in the Liver: OAA is a crucial intermediate in the Citric Acid Cycle (TCA cycle) that combines with Acetyl-CoA to form citrate.
- During fasting, OAA is diverted to gluconeogenesis (glucose synthesis) in the liver to maintain blood glucose levels.
- This depletion of OAA means that Acetyl-CoA cannot efficiently enter the TCA cycle.
- High Glucagon/Insulin Ratio: Glucagon promotes fatty acid mobilization and gluconeogenesis, further contributing to the conditions favoring ketogenesis.
- Clinical States: Starvation/Fasting, Uncontrolled Diabetes Mellitus (Type 1), Low Carbohydrate, High-Fat Diets (Ketogenic Diets).
In essence, ketogenesis is a response to an oversupply of Acetyl-CoA (from fat breakdown) and an undersupply of OAA (due to gluconeogenesis) in the liver.
Synthesis of Ketone Bodies (Ketogenesis) in the Liver
Ketogenesis occurs exclusively in the mitochondrial matrix of liver cells.
The Three Ketone Bodies:
- Acetoacetate: The primary ketone body produced.
- β-Hydroxybutyrate: Formed by the reduction of acetoacetate.
- Acetone: A volatile byproduct of acetoacetate breakdown, produced in smaller quantities and excreted via breath.
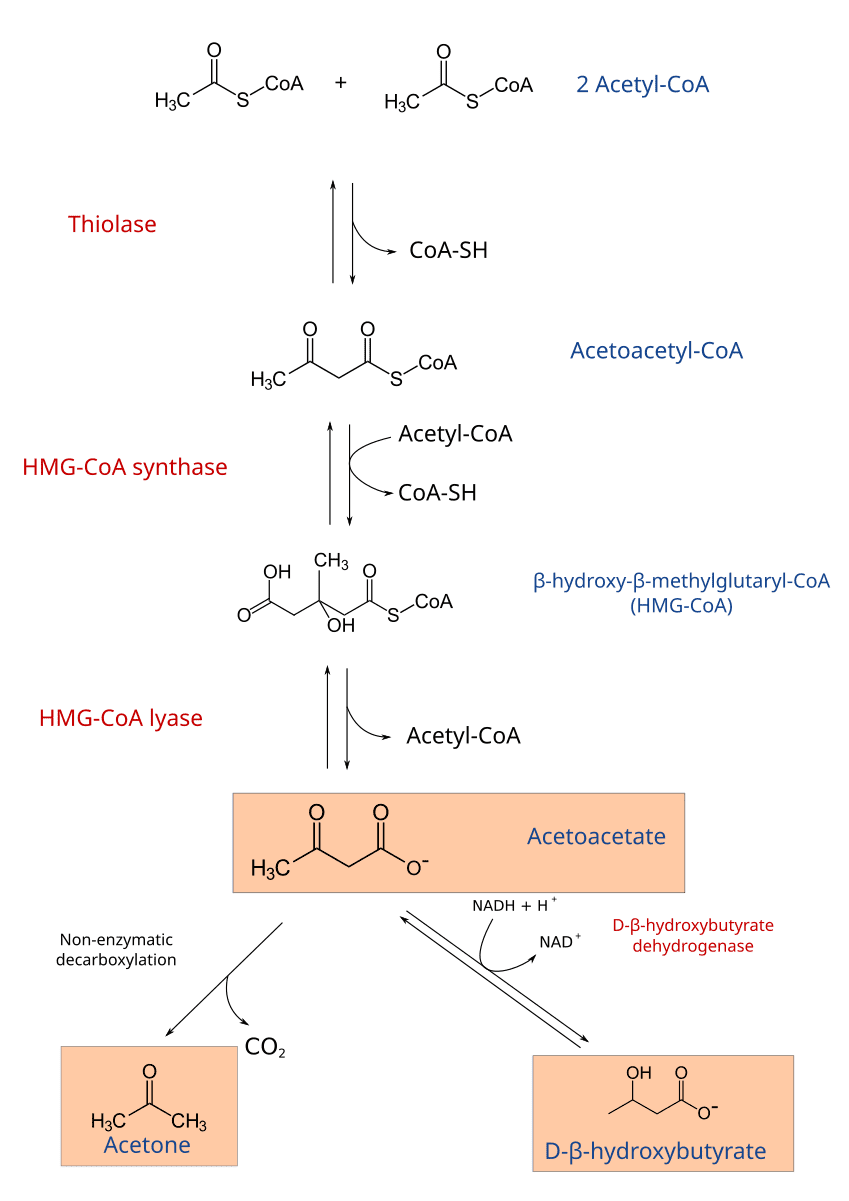
Steps of Ketogenesis:
-
1. Condensation of Two Acetyl-CoA Molecules:
- Enzyme: Thiolase (the reverse reaction of the last step of β-oxidation).
- Reaction:
2 Acetyl-CoA → Acetoacetyl-CoA + CoA-SH
-
2. Condensation with a Third Acetyl-CoA:
- Enzyme: HMG-CoA Synthase (Hydroxymethylglutaryl-CoA Synthase).
- Reaction:
Acetoacetyl-CoA + Acetyl-CoA + H₂O → β-hydroxy-β-methylglutaryl-CoA (HMG-CoA) + CoA-SH - Note: This is the rate-limiting step of ketogenesis.
-
3. Cleavage of HMG-CoA:
- Enzyme: HMG-CoA Lyase.
- Reaction:
HMG-CoA → Acetoacetate + Acetyl-CoA - This reaction produces the first ketone body, acetoacetate.
-
4. Interconversion and Breakdown of Acetoacetate:
- Acetoacetate can be reduced to β-hydroxybutyrate.
- Enzyme: β-hydroxybutyrate Dehydrogenase.
- Reaction:
Acetoacetate + NADH + H⁺ ⇌ β-Hydroxybutyrate + NAD⁺
- Acetoacetate can also spontaneously decarboxylate to Acetone (
Acetoacetate → Acetone + CO₂).
- Acetoacetate can be reduced to β-hydroxybutyrate.
Utilization (Ketolysis) of Ketone Bodies by Extrahepatic Tissues
Ketone bodies are water-soluble and can be transported via the bloodstream to peripheral tissues, which then convert them back into Acetyl-CoA for energy. The liver cannot utilize ketone bodies because it lacks a key enzyme for ketolysis.
Tissues that use Ketone Bodies: Brain, heart, skeletal muscle, renal cortex.
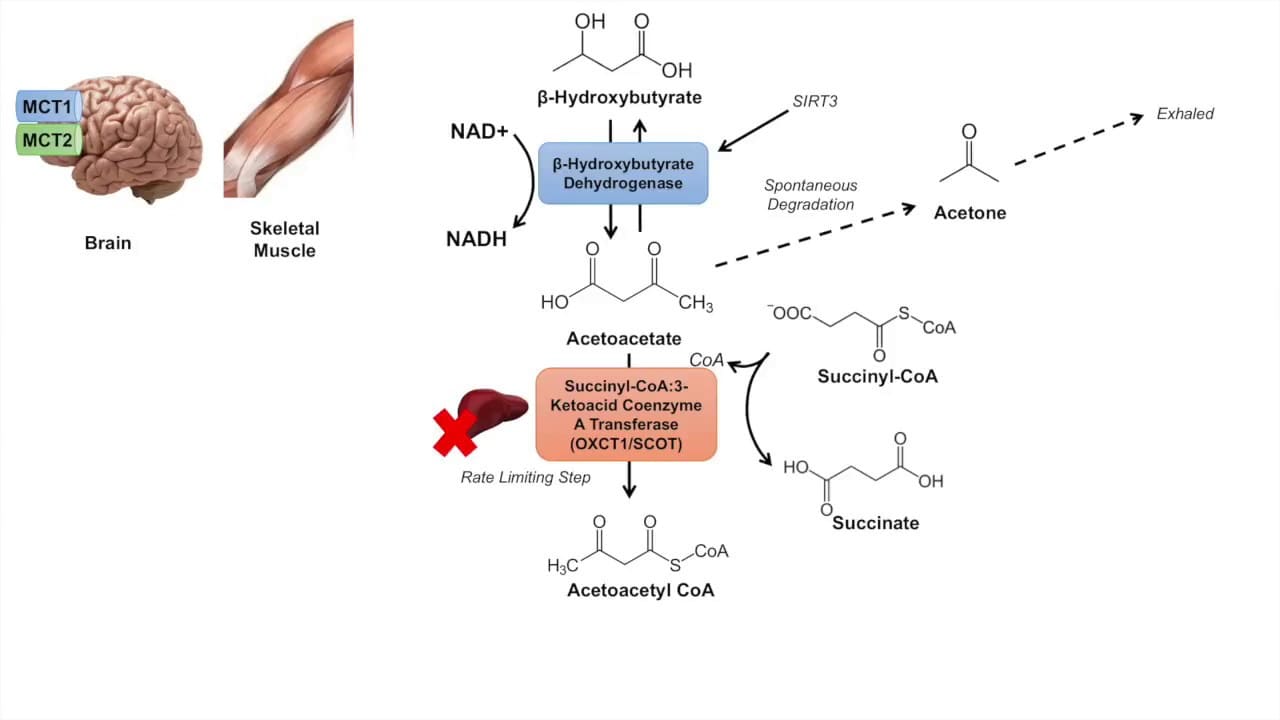
Steps of Ketolysis (Example: in the Brain/Muscle):
-
1. Conversion of β-Hydroxybutyrate to Acetoacetate:
- Enzyme: β-hydroxybutyrate Dehydrogenase.
- Reaction:
β-Hydroxybutyrate + NAD⁺ → Acetoacetate + NADH + H⁺
-
2. Activation of Acetoacetate:
- Enzyme: β-ketoacyl-CoA Transferase (also called Thiophorase).
- Reaction:
Acetoacetate + Succinyl-CoA → Acetoacetyl-CoA + Succinate - Crucial: This enzyme is absent in the liver, which is why the liver produces but cannot utilize ketone bodies.
-
3. Cleavage of Acetoacetyl-CoA:
- Enzyme: Thiolase.
- Reaction:
Acetoacetyl-CoA + CoA-SH → 2 Acetyl-CoA
The 2 molecules of Acetyl-CoA produced can then enter the Citric Acid Cycle to generate ATP.
Clinical Significance of Ketogenesis
The production and utilization of ketone bodies are normally well-regulated. However, imbalances can lead to serious clinical conditions.
- Physiological Ketosis: A normal and beneficial state that occurs during prolonged fasting, starvation, or a strict ketogenic diet. Ketone bodies provide a crucial fuel source, especially for the brain, preserving muscle protein.
- Pathological Ketosis (Ketoacidosis):
- Diabetic Ketoacidosis (DKA): This is a life-threatening complication of Type 1 Diabetes Mellitus.
- Cause: Absolute or severe relative insulin deficiency combined with elevated glucagon levels.
- Mechanism: Lack of insulin means cells cannot take up glucose, leading to severe hyperglycemia. Simultaneously, high glucagon promotes massive lipolysis and unchecked ketogenesis in the liver.
- Consequences: The rapid and excessive production of acidic ketone bodies overwhelms the body's buffering capacity, leading to a significant drop in blood pH (acidosis), dehydration, electrolyte imbalances, and potentially coma and death if untreated.
- Acetone: The increased production of acetoacetate leads to increased spontaneous decarboxylation to acetone, giving the breath of DKA patients a characteristic "fruity" odor.
- Alcoholic Ketoacidosis: Can occur in chronic alcoholics, often exacerbated by poor nutrition. Alcohol metabolism produces excess NADH, which shifts OAA to malate and inhibits gluconeogenesis, leading to a similar state of excessive ketogenesis and acidosis.
- Diabetic Ketoacidosis (DKA): This is a life-threatening complication of Type 1 Diabetes Mellitus.
Fatty Acid Synthesis (Lipogenesis)
When the body has an abundance of energy, especially from a diet rich in carbohydrates, it converts excess glucose into fatty acids for long-term storage as triacylglycerols. This process is called lipogenesis.
Overview and Key Tissues
- Definition: The metabolic pathway that synthesizes fatty acids from acetyl-CoA.
- Primary Precursor: Acetyl-CoA, which is largely derived from carbohydrate metabolism (pyruvate oxidation).
- Location: Primarily in the cytosol of cells.
- Major Sites:
Liver: The most active site of fatty acid synthesis.Adipose Tissue: Also synthesizes fatty acids.Lactating Mammary Glands: Synthesize fatty acids for milk production.
- Main Product: Palmitate (16:0), a saturated 16-carbon fatty acid.
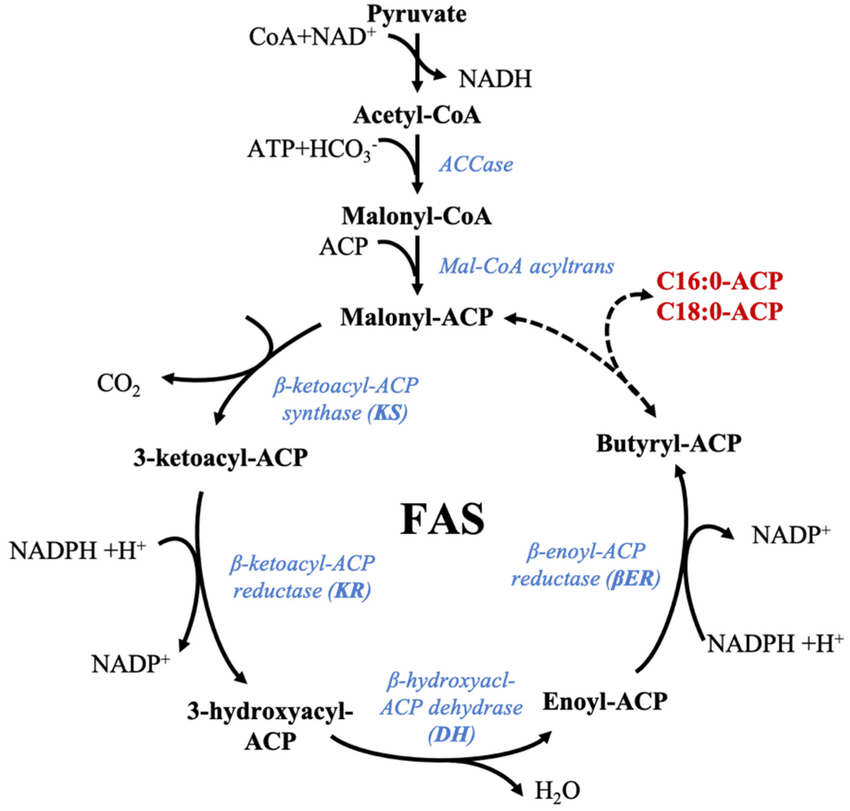
Key Steps and Enzymes in Fatty Acid Synthesis
Fatty acid synthesis is essentially a reversal of β-oxidation, but it uses different enzymes, occurs in a different cellular compartment, and employs a different electron donor.
A. Transport of Acetyl-CoA from Mitochondria to Cytosol:
- Problem: Acetyl-CoA is produced in the mitochondrial matrix, but synthesis occurs in the cytosol. The inner mitochondrial membrane is impermeable to Acetyl-CoA.
- Solution: The Citrate Shuttle
- Condensation: Acetyl-CoA combines with oxaloacetate (OAA) in the mitochondrial matrix to form citrate (catalyzed by Citrate Synthase).
- Transport: Citrate is transported across the inner mitochondrial membrane into the cytosol.
- Cleavage: In the cytosol, citrate is cleaved back into Acetyl-CoA and OAA by ATP Citrate Lyase. This step requires ATP.
Citrate + ATP + CoA-SH → Acetyl-CoA + OAA + ADP + Pi - Recycling OAA: The cytosolic OAA is converted to malate and then pyruvate (producing NADPH in the process via malic enzyme) before returning to the mitochondria.
B. Carboxylation of Acetyl-CoA to Malonyl-CoA:
- Enzyme: Acetyl-CoA Carboxylase (ACC).
- Reaction:
Acetyl-CoA + HCO₃⁻ + ATP → Malonyl-CoA + ADP + Pi - Significance: This is the rate-limiting and committed step of fatty acid synthesis.
- Requirements: Biotin and ATP.
C. The Fatty Acid Synthase Complex:
Synthesis is carried out by a multi-enzyme complex called Fatty Acid Synthase (FAS). It contains seven different enzymatic activities and an acyl carrier protein (ACP).
- Electron Donor: NADPH (not NADH or FADH₂).
Steps of the FAS Cycle (Repeated 7 Times):
Each cycle adds a two-carbon unit from Malonyl-CoA and involves four steps:
- Condensation: The growing fatty acyl chain condenses with malonyl-ACP, releasing CO₂. (Enzyme: β-ketoacyl-ACP Synthase).
- Reduction (by NADPH): The β-keto group is reduced to a β-hydroxy group. (Enzyme: β-ketoacyl-ACP Reductase).
- Dehydration: Water is removed, creating a double bond. (Enzyme: β-hydroxyacyl-ACP Dehydratase).
- Reduction (by NADPH): The double bond is reduced, resulting in a saturated acyl-ACP chain that is two carbons longer. (Enzyme: Enoyl-ACP Reductase).
After 7 cycles, the 16-carbon palmitoyl-ACP is formed and then released as free palmitate by a Thioesterase.
Summary of Palmitate Synthesis:
Overall Reaction: 8 Acetyl-CoA + 7 ATP + 14 NADPH → Palmitate + 8 CoA + 7 ADP + 7 Pi + 14 NADP⁺ + 6 H₂O
Regulation of Fatty Acid Synthesis
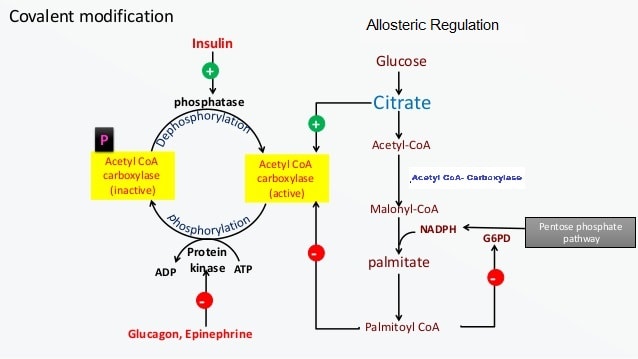
A. Short-Term Regulation (of ACC):
- Allosteric Activators: Citrate. High levels of citrate indicate excess energy and activate ACC.
- Allosteric Inhibitors: Long-Chain Fatty Acyl-CoAs. High levels of the end-product inhibit ACC.
- Covalent Modification:
- Dephosphorylation (Activation): Insulin activates a phosphatase that dephosphorylates and activates ACC.
- Phosphorylation (Inhibition): Glucagon and Epinephrine activate PKA, which phosphorylates and inactivates ACC. AMP-activated protein kinase (AMPK) also inactivates ACC when cellular energy is low.
B. Long-Term Regulation (Gene Expression):
- Dietary Factors: High-carbohydrate, low-fat diets increase the synthesis of ACC and FAS enzymes. Fasting or high-fat diets decrease their synthesis.
- Hormonal Factors: Insulin increases the synthesis of enzymes for fatty acid synthesis.
Elongation and Desaturation of Fatty Acids
Once palmitate (16:0) is synthesized, it can be further modified:
- Elongation: Occurs primarily in the endoplasmic reticulum (ER). Adds two carbons at a time, using Malonyl-CoA and NADPH, to produce stearate (18:0) and other longer fatty acids.
- Desaturation: Occurs in the ER. Introduces double bonds into saturated fatty acids.
- Enzymes: Fatty Acyl-CoA Desaturases, which require O₂, NADH (or NADPH), and cytochrome b5.
- Limitations: Mammals can introduce double bonds at Δ9, Δ6, and Δ5 positions but cannot introduce double bonds beyond Δ9. This is why linoleic acid (Δ9,12) and α-linolenic acid (Δ9,12,15) are essential fatty acids.
Regulation and Interplay with Other Pathways
The metabolism of fatty acids is not an isolated process; it is intricately woven into the overall metabolic fabric of the cell and the organism. Regulation ensures that energy is stored when abundant and mobilized when needed, all while maintaining metabolic homeostasis.
Hormonal Regulation
Hormones are the primary messengers that coordinate fatty acid metabolism across different tissues in response to the body's energy status.
A. Insulin (Hormone of the "Fed" State):
- Effect on Lipogenesis (Fatty Acid Synthesis):
Promotes.- Increases glucose uptake into adipocytes and liver.
- Activates Pyruvate Dehydrogenase, increasing Acetyl-CoA supply.
- Activates Acetyl-CoA Carboxylase (ACC) by dephosphorylation (reducing its Km for citrate).
- Induces gene expression of ACC and Fatty Acid Synthase (FAS).
- Increases the activity of Lipoprotein Lipase (LPL) in adipose tissue, facilitating uptake of dietary TAGs.
- Effect on Lipolysis (Fatty Acid Breakdown):
Inhibits.- Decreases cAMP levels, leading to dephosphorylation and inactivation of Hormone-Sensitive Lipase (HSL).
- Overall: Insulin promotes energy storage in the form of glycogen and triacylglycerols.
B. Glucagon (Hormone of the "Fasted" State):
- Effect on Lipogenesis: Inhibits. Inactivates ACC by phosphorylation (via PKA).
- Effect on Lipolysis: Promotes. Increases cAMP levels, leading to phosphorylation and activation of HSL.
- Overall: Glucagon promotes the mobilization of stored energy, including fatty acids.
C. Epinephrine and Norepinephrine (Catecholamines - "Fight or Flight" Hormones):
- Effect on Lipolysis: Potent stimulators. Bind to adrenergic receptors on adipocytes, leading to increased cAMP and activation of HSL via PKA.
- Overall: Rapidly mobilizes fatty acids for immediate energy needs during stress.
D. Thyroid Hormones:
Generally increase metabolic rate, which can indirectly affect fatty acid metabolism by increasing both synthesis and breakdown, depending on the overall energy balance.
Allosteric and Covalent Regulation
Beyond hormones, specific molecules within metabolic pathways can directly activate or inhibit key enzymes.
A. Regulation of Acetyl-CoA Carboxylase (ACC) - Key for Synthesis:
- Allosteric Activator:
Citrate(high levels indicate abundant energy and Acetyl-CoA). - Allosteric Inhibitor: Long-chain fatty acyl-CoAs (product inhibition).
- Covalent Modification: Phosphorylation (by PKA, AMPK) inactivates; dephosphorylation (by insulin-activated phosphatase) activates.
B. Regulation of Carnitine Palmitoyltransferase I (CPT-I) - Key for Oxidation:
- Allosteric Inhibitor:
Malonyl-CoA. - This is a crucial point of reciprocal regulation: When fatty acid synthesis is active (high
Malonyl-CoA), fatty acid oxidation is inhibited at the entry point to the mitochondria. This prevents a "futile cycle".
C. Regulation of Hormone-Sensitive Lipase (HSL) - Key for Mobilization:
- Covalent Modification: Phosphorylation (by PKA) activates; dephosphorylation (by insulin-activated phosphatase) inactivates.
Transcriptional (Gene Expression) Regulation
Long-term adaptation to dietary and hormonal changes involves altering the amount of enzymes present.
- Insulin: Upregulates the synthesis of enzymes for lipogenesis (ACC, FAS, ATP citrate lyase).
- Fasting/Starvation: Downregulates the synthesis of lipogenic enzymes and upregulates enzymes for fatty acid oxidation.
- PPARs (Peroxisome Proliferator-Activated Receptors): These are nuclear receptors that act as transcription factors. For example, PPARα is activated by fatty acids and promotes the expression of genes involved in fatty acid oxidation.
Interplay with Other Metabolic Pathways
A. Fatty Acid-Carbohydrate Interplay (The Glucose-Fatty Acid Cycle / Randle Cycle):
- In the Fed State: High glucose leads to insulin release, promoting glucose utilization and lipogenesis.
- In the Fasted State: Low glucose leads to glucagon release, promoting lipolysis. The increased fatty acids and their oxidation products (
Acetyl-CoA,NADH,citrate) inhibit glucose utilization in peripheral tissues, sparing glucose for the brain.- High
Acetyl-CoAinhibits Pyruvate Dehydrogenase. - High
citrateinhibits PFK-1 (Phosphofructokinase-1). - High
NADH/NAD⁺ratio also inhibits various steps in carbohydrate metabolism.
- High
- Overall: There's a reciprocal relationship: high fatty acid oxidation inhibits glucose oxidation, and vice versa.
B. Fatty Acid-Protein Interplay:
- Some amino acids can be converted to
Acetyl-CoAfor fatty acid synthesis. - During starvation, protein breakdown becomes a more significant source of energy and gluconeogenic precursors.
C. Fatty Acid-Ketone Body Interplay:
When fatty acid oxidation is high and OAA is diverted to gluconeogenesis, excess Acetyl-CoA is converted into ketone bodies in the liver, serving as an alternative fuel for extrahepatic tissues, particularly the brain.
D. Connection to Cholesterol Synthesis:
Acetyl-CoA is the sole precursor for cholesterol synthesis. HMG-CoA (an intermediate in ketogenesis) is also an intermediate in cholesterol synthesis.
Biochemistry: Fatty Acid Metabolism
Test your knowledge with these 40 questions.
Fatty Acid Metabolism Quiz
Question 1/40
Quiz Complete!
Here are your results, .
Your Score
38/40
95%