Carbohydrate Metabolism: Glycolysis
Glycolysis: The Embden-Meyerhof Pathway
Building upon our understanding of bioenergetics, we now delve into Glycolysis, also known as the Embden-Meyerhof Pathway (EMP). This fundamental metabolic pathway is the initial step in breaking down glucose to generate energy in nearly all living organisms.
Glycolysis (from Greek "glykys" = sweet, "lysis" = splitting) is the process where one molecule of glucose (a 6-carbon sugar) is broken down into two molecules of pyruvate (a 3-carbon compound). This process releases a small amount of energy, which is captured as ATP and NADH.
- Location: All the reactions of glycolysis occur in the cytoplasm of the cell. This means it doesn't require mitochondria.
- Key Roles:
- Energy for Mitochondria-Lacking Tissues: It's the primary way tissues without mitochondria (like red blood cells, cornea, and lens) produce ATP.
- Brain's Energy Source: The brain relies heavily on glucose, and glycolysis is its initial step in energy extraction.
- Anaerobic vs. Aerobic Fates:
- Without Oxygen (Anaerobic): Pyruvate is converted to lactate, providing a quick, albeit limited, energy supply (2 net ATP per glucose).
- With Oxygen (Aerobic): Pyruvate enters the mitochondria for further breakdown in the Citric Acid Cycle and Oxidative Phosphorylation, which yields a much larger amount of ATP.
Energy Yield and Thermodynamics of Glycolysis
Glycolysis is an energy-releasing (exergonic) pathway.
Overall Chemical Transformation:
This reaction generates energy that is used to produce ATP:
Free Energy Changes (Standard Biological Conditions, ΔG°'):
- Energy released from glucose conversion to pyruvate:
ΔG°' = -146 kJ/mol - Energy required to form 2 ATP:
2 × (30.5 kJ/mol) = 61 kJ/mol - Overall Net Free Energy Change:
ΔG°' (overall) = -146 kJ/mol + 61 kJ/mol = -85 kJ/mol
Thermodynamic Summary:
The significantly negative overall ΔG°' indicates that glycolysis is an exergonic reaction that proceeds spontaneously and is largely irreversible under standard conditions.
Glycolysis releases only a small fraction of the total potential energy stored in glucose. Specifically:
"5.2% of the total free energy that can be released by glucose is released in glycolysis."
The complete oxidation of glucose yields much more energy (ΔG°' = -2840 kJ/mol), meaning the majority of glucose's energy remains in pyruvate and NADH, awaiting further aerobic processing.
Fates of Glucose in Living Systems
Once glucose enters a cell, it has 4 primary metabolic fates, depending on the organism's immediate needs:
- Storage: Glucose can be linked to form large storage polymers like Glycogen in animals or Starch in plants.
- Structural Synthesis: Glucose derivatives can be used to synthesize polysaccharides that form structural components, such as the extracellular matrix.
- Oxidation via Pentose Phosphate Pathway (PPP): Glucose is converted to Ribose 5-phosphate. This pathway is vital for producing NADPH (for biosynthesis and protecting from oxidative damage) and Ribose 5-phosphate (for synthesizing nucleotides like DNA and RNA).
- Oxidation via Glycolysis (Energy Production): Glucose is broken down to Pyruvate, serving as the initial step for ATP production.
Historical Discovery of Glycolysis
The elucidation of glycolysis was a monumental achievement, marking it as one of the first and "oldest" metabolic pathways to be fully understood.
- Louis Pasteur (1854-1864): Observed that fermentation was caused by microorganisms. His "Pasteur effect" noted that organisms use less glucose in the presence of oxygen because aerobic respiration is far more efficient.
- Eduard Buchner (1897): Revolutionized biochemistry by demonstrating that yeast extracts, even without living cells, could carry out fermentation, proving that enzymes were responsible.
- Harden and Young (1905): Made two key discoveries: inorganic phosphate is essential for fermentation, and yeast extracts could be separated into heat-stable small molecules ("Co-zymase," later identified as NAD+, ATP, ADP) and heat-labile protein enzymes ("Zymase").
- By 1940: Through the combined efforts of many scientists, including Gustav Embden, Otto Meyerhof, and Jacob Parnas, the complete step-by-step pathway of glycolysis was definitively established.
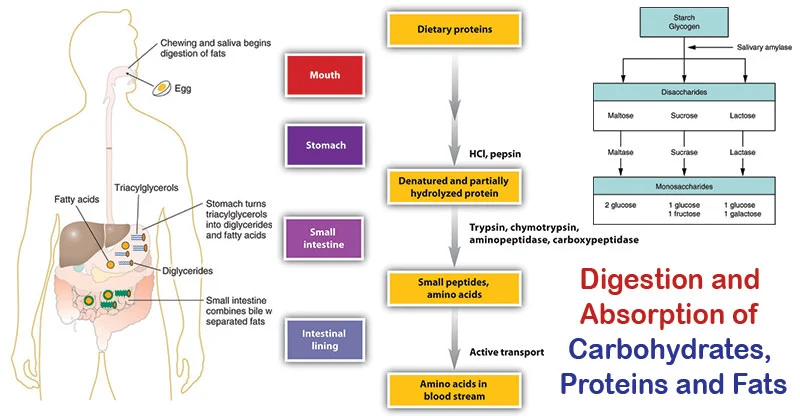
Digestion and Absorption of Dietary Carbohydrates
Before carbohydrates can be used by the body, complex forms (polysaccharides and disaccharides) must be broken down into monosaccharides for absorption. This process begins in the mouth and continues in the small intestine.
1. Digestion
Digestion involves the enzymatic hydrolysis of glycosidic bonds.
-
In the Mouth:
- Mechanical digestion (chewing).
- Salivary alpha-amylase (Ptyalin): Begins the breakdown of starch into smaller polysaccharides (dextrins) and some maltose. It's inactivated by stomach acid.
- In the Stomach: No significant carbohydrate digestion occurs here due to the acidic environment.
-
In the Small Intestine: This is where the bulk of carbohydrate digestion takes place.
- Pancreatic alpha-amylaseme: Continues breaking down starch and dextrins into maltose and other small polymers.
- Brush Border Enzymes: Located on the microvilli of intestinal cells, these are responsible for the final breakdown into monosaccharides.
- Maltase: Hydrolyzes maltose → two glucose molecules.
- Sucrase: Hydrolyzes sucrose → one glucose and one fructose.
- Lactase: Hydrolyzes lactose → one glucose and one galactose.
- Alpha-dextrinase (Isomaltase): Hydrolyzes alpha-1,6 bonds in limit dextrins, releasing glucose.
The end products are almost exclusively monosaccharides: glucose, fructose, and galactose.
2. Absorption
Monosaccharides are absorbed by intestinal epithelial cells (enterocytes) and then transported into the bloodstream.
-
Glucose and Galactose Absorption:
- Primarily absorbed by secondary active transport via the SGLT1 (Sodium-Glucose Cotransporter 1) protein. This requires energy and co-transports Na⁺ ions.
- From the enterocyte, they exit into the bloodstream via facilitated diffusion through the GLUT2 transporter.
-
Fructose Absorption:
- Absorbed solely by facilitated diffusion via the GLUT5 transporter. This does not require energy.
- From the enterocyte, it also exits into the bloodstream via the GLUT2 transporter.
3. Transport to the Liver
Once absorbed, these monosaccharides enter the hepatic portal vein, which carries them directly to the liver. The liver is the primary site for fructose and galactose metabolism, converting them into glucose or its intermediates.
Clinical Significance:
- Lactose Intolerance: A deficiency of the enzyme lactase, leading to maldigestion of lactose.
- Pancreatic Insufficiency: Conditions like cystic fibrosis can lead to maldigestion of starch.
- SGLT1 Deficiency: A rare genetic disorder where glucose and galactose cannot be absorbed.
Fates of Absorbed Monosaccharides (Especially Glucose)
After absorption, our monosaccharides (primarily glucose) enter the bloodstream. The body then has several crucial "fates" or pathways for this glucose, depending on energy needs and hormonal signals.
Visualizing the "Fates":
Imagine glucose as a central hub. From this hub, it can be directed down different "roads":
- Road 1: "Burn it for immediate power!" (Glycolysis → TCA → ETC)
- Road 2: "Store it for a quick pick-me-up!" (Glycogenesis)
- Road 3: "Pack it away for a rainy day!" (Conversion to Fat)
- Road 4: "Build other essential parts!" (Pentose Phosphate Pathway)
Energy Production (Oxidation):
- Goal: To generate ATP.
- Pathways: Glycolysis → Pyruvate Oxidation → TCA Cycle → Oxidative Phosphorylation.
- When: Continuously in most cells, especially during high energy demand.
Storage as Glycogen (Glycogenesis):
- Goal: To store excess glucose for later use.
- Where: Primarily in the liver and skeletal muscles.
- When: When blood glucose is high (e.g., after a meal), stimulated by insulin.
Conversion to Fat (Lipogenesis):
- Goal: To store excess energy in a long-term form when glycogen stores are full.
- Pathways: Glucose is converted to Acetyl-CoA, which is then used for fatty acid synthesis and stored as triglycerides in adipose tissue.
- When: When carbohydrate intake consistently exceeds energy needs.
Formation of Other Biomolecules (e.g., via Pentose Phosphate Pathway):
- Goal: To provide precursors for other essential molecules.
- Pathway: Pentose Phosphate Pathway (PPP) / Hexose Monophosphate Shunt (HMP Shunt):
- Produces NADPH: Crucial for biosynthesis (e.g., fatty acids) and protecting cells from oxidative stress.
- Produces Ribose-5-phosphate: A key component of nucleotides (DNA, RNA) and coenzymes (ATP, NADH).
- When: Continuously in cells with high demand for NADPH (e.g., liver, adipose tissue) or nucleotide synthesis.
Stages of Glycolysis: An Overview
Glycolysis proceeds through a sequence of ten enzyme-catalyzed reactions, typically divided into two main stages:
Stage 1: Energy Investment (Reactions 1-5)
This initial stage is a preparatory phase where the glucose molecule is modified and split. It requires an input of energy.
Key events:
- Phosphorylation of Glucose: Glucose is phosphorylated (a phosphate group is added) to trap it within the cell and increase its reactivity.
- Isomerization and Second Phosphorylation: The phosphorylated glucose is rearranged and then phosphorylated again, forming a doubly phosphorylated fructose molecule.
- Cleavage: This 6-carbon molecule is then cleaved into two molecules of glyceraldehyde-3-phosphate (a 3-carbon compound).
Energy Cost: This stage involves an investment of two molecules of ATP. These ATP molecules are consumed to add the phosphate groups, effectively "priming" the molecule for later energy extraction.
Stage 2: Energy Payoff (Reactions 6-10)
In this stage, the two glyceraldehyde-3-phosphate molecules are converted into pyruvate, generating ATP and NADH.
Key events:
- Oxidation and Phosphorylation: The two molecules of glyceraldehyde-3-phosphate undergo oxidation and further phosphorylation.
- ATP Production: Energy released is used to generate ATP directly through substrate-level phosphorylation.
- Formation of Pyruvate: The final product is two molecules of pyruvate.
Energy Gain: This stage produces a total of four molecules of ATP and two molecules of NADH (nicotinamide adenine dinucleotide, an electron carrier) per glucose molecule.
Net Energy Yield of Glycolysis
Considering both stages, the overall net gain from glycolysis per molecule of glucose is:
- Net ATP: 4 ATP produced - 2 ATP invested = 2 Net ATP
- Net NADH: 2 NADH (These will be used to generate more ATP later in aerobic respiration).
VI. Importance of Phosphorylated Intermediates
The fact that many intermediates in glycolysis are phosphorylated serves several critical purposes:
- Trapping within the Cell: The addition of a negatively charged phosphate group makes these intermediates hydrophilic and unable to easily cross the nonpolar cell membrane. This inhibits their diffusion out of the cell, ensuring they remain available for metabolic processing.
- Conservation of Free Energy: The phosphate group forms a "high-energy" bond in certain intermediates. The energy stored in these bonds can be directly transferred to ADP to form ATP during substrate-level phosphorylation, as seen in Stage 2 of glycolysis.
- Facilitation of Catalysis: The phosphate groups act as binding sites for enzymes. They help position the substrate correctly in the active site and contribute to the overall binding energy, thus facilitating the enzyme-catalyzed reactions. The negative charges also alter the electronic configuration of the molecule, making it more reactive.
Click Here To play the Game of Glycolysis
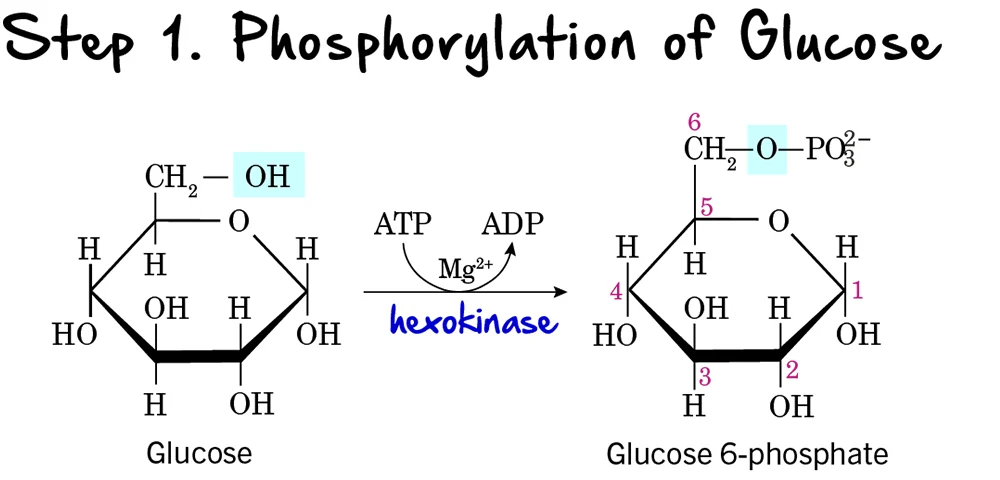
Glycolysis: Step 1 - Phosphorylation of Glucose
The first step in glycolysis is a crucial preparatory reaction, where glucose is activated and trapped within the cell.
Reaction:
Glucose is phosphorylated on its carbon 6 (C6) hydroxyl group to form Glucose 6-phosphate (G6P). This reaction consumes one molecule of ATP.
Key Features of Step 1:
-
Enzyme: The phosphorylation is catalyzed by kinases, which are enzymes that transfer a phosphate group from ATP.
- Hexokinase: Found in most tissues. It has a high affinity for glucose, meaning it can efficiently phosphorylate glucose even at low concentrations. It is inhibited by its product, glucose-6-phosphate.
-
Glucokinase: Primarily found in the liver and pancreatic beta cells. It has a lower affinity for glucose, acting only when blood glucose levels are high. It is not inhibited by glucose-6-phosphate, allowing the liver to continue taking up glucose. Both enzymes require
Mg²⁺as a cofactor.
- Intermediate Formed: Glucose 6-phosphate
- ATP Change: -1 ATP (One ATP molecule is consumed). This is the first "investment" in the energy-investment phase.
Purpose of Phosphorylation:
- Traps Glucose in the Cell: The addition of a negatively charged phosphate group prevents glucose 6-phosphate from easily crossing the cell membrane. Once phosphorylated, glucose is effectively "locked" inside the cell.
- Activates Glucose: The phosphate group makes glucose more reactive and unstable, priming it for subsequent enzymatic reactions.
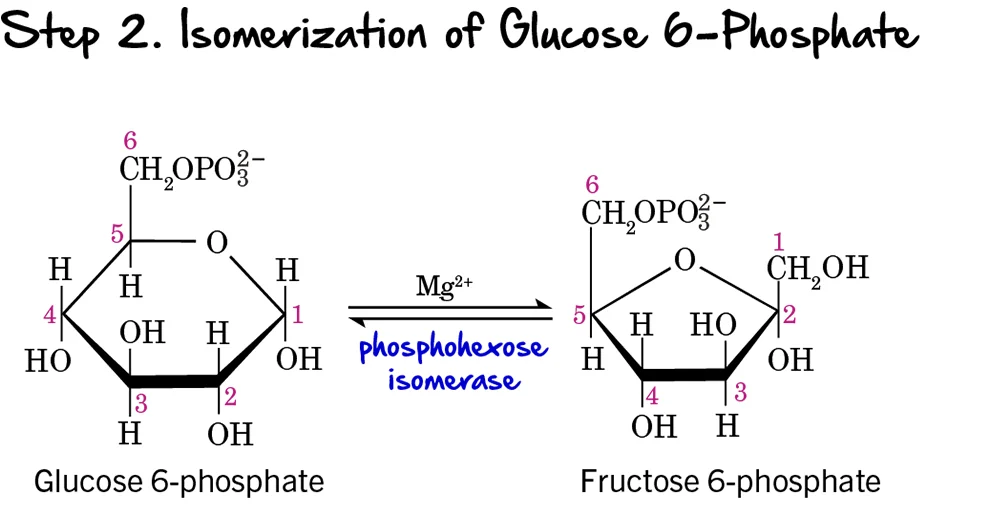
Step 2 - Isomerization of Glucose 6-Phosphate
Following the phosphorylation of glucose, the next step involves a rearrangement of the molecule from an aldose to a ketose.
Reaction:
Glucose 6-phosphate (an aldose) is isomerized (rearranged) into Fructose 6-phosphate (a ketose). This reaction is reversible.
Key Features of Step 2:
-
Enzyme: The reaction is catalyzed by Phosphohexose Isomerase (also known as Phosphoglucose Isomerase or PGI). It requires
Mg²⁺as a cofactor. - Intermediate Formed: Fructose 6-phosphate
- ATP Change: 0 ATP (No ATP is consumed or produced in this step).
Purpose of Isomerization:
This isomerization is crucial because it sets up the molecule for the next two steps in glycolysis:
- It creates a primary alcohol group at carbon 1 (C1) of fructose 6-phosphate, which can then be phosphorylated in the next step.
- It prepares the molecule for symmetric cleavage in a later step (Step 4), allowing it to be split into two 3-carbon units. If glucose 6-phosphate were cleaved directly, it would result in unequal 2-carbon and 4-carbon fragments.
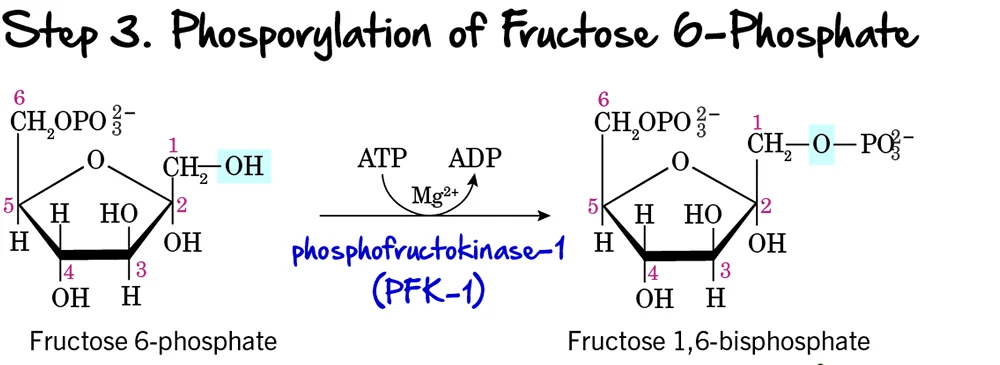
Step 3 - Phosphorylation of Fructose 6-Phosphate
This is a critical and highly regulated step in glycolysis, often considered the "committed step" of the pathway.
Reaction:
Fructose 6-phosphate undergoes a second phosphorylation, this time at its carbon 1 (C1) hydroxyl group, to form Fructose 1,6-bisphosphate. This reaction consumes another molecule of ATP.
Key Features of Step 3:
-
Enzyme: The enzyme catalyzing this reaction is Phosphofructokinase-1 (PFK-1). This is a crucial enzyme and a major regulatory point. It requires ATP as the phosphate donor and
Mg²⁺as a cofactor. - Intermediate Formed: Fructose 1,6-bisphosphate
- ATP Change: -1 ATP (Another ATP molecule is invested, bringing the total to 2 ATP).
Purpose of this Step:
- Commitment to Glycolysis: The formation of Fructose 1,6-bisphosphate is the committed step. Once formed, this molecule is generally destined to proceed through the rest of the glycolytic pathway.
- Preparation for Cleavage: Having phosphate groups on both ends (C1 and C6) is essential for the symmetrical cleavage that occurs in the next step.
Regulation of PFK-1:
PFK-1 is a key control point because its activity dictates the overall rate of glycolysis. It is allosterically regulated:
-
Activation:
- High AMP (Adenosine Monophosphate): Indicates low cellular energy, so PFK-1 is activated to increase ATP production.
- Fructose 2,6-bisphosphate: A potent allosteric activator, signaling high levels of available glucose.
-
Inhibition:
- High ATP: Indicates ample cellular energy. ATP binds to an allosteric site on PFK-1, reducing its activity.
- High Citrate: An intermediate of the Krebs Cycle, indicating that energy precursors are abundant, thus signaling to slow glycolysis.
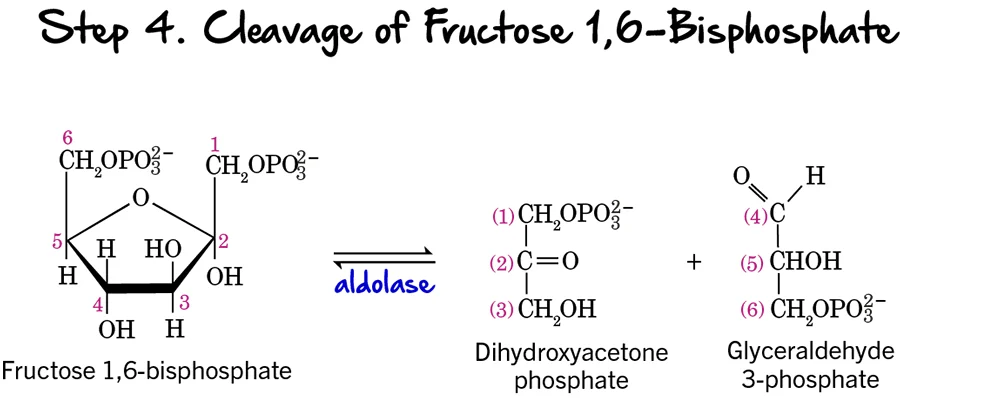
Step 4 - Cleavage of Fructose 1,6-bisphosphate
After two phosphorylation steps and an isomerization, the 6-carbon sugar is now ready to be split into two 3-carbon molecules, marking the true "lysis" of glycolysis.
Reaction:
Fructose 1,6-bisphosphate (a 6-carbon sugar) is cleaved into two distinct 3-carbon phosphorylated sugars:
- Glyceraldehyde 3-phosphate (GAP), an aldose sugar.
- Dihydroxyacetone phosphate (DHAP), a ketose sugar.
Key Features of Step 4:
- Enzyme: The enzyme catalyzing this reversible cleavage is Aldolase. The name refers to its ability to catalyze an aldol cleavage reaction.
- Intermediates Formed: Glyceraldehyde 3-phosphate (GAP) and Dihydroxyacetone phosphate (DHAP).
- ATP Change: 0 ATP (No ATP is consumed or produced).
Purpose of the Cleavage:
This step is crucial because it takes the single 6-carbon sugar and converts it into two 3-carbon molecules. These two molecules will then proceed through the second, "energy payoff" stage. The previous isomerization to fructose 6-phosphate (Step 2) was essential to enable this symmetrical cleavage into two triose phosphates, making the rest of the pathway more efficient.
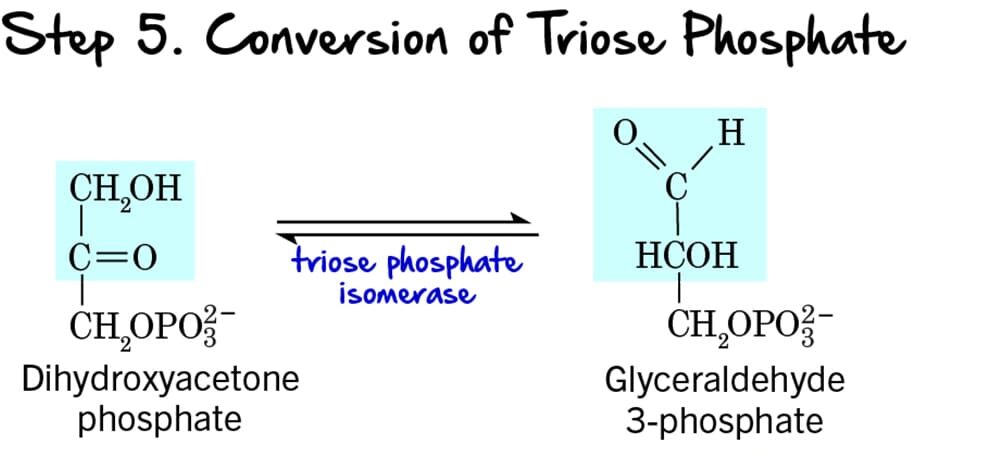
Step 5 - Interconversion of Triose Phosphates
Following the cleavage of Fructose 1,6-bisphosphate (Step 4), two different 3-carbon sugars are produced: Dihydroxyacetone phosphate (DHAP) and Glyceraldehyde 3-phosphate (GAP). However, only GAP can directly proceed into the next steps of glycolysis. This step ensures that both molecules can be utilized.
Reaction:
Dihydroxyacetone phosphate (DHAP), a ketose, is reversibly isomerized into Glyceraldehyde 3-phosphate (GAP), an aldose.
Key Features of Step 5:
- Enzyme: The enzyme catalyzing this reversible isomerization is Triose Phosphate Isomerase (TPI). This enzyme is remarkably efficient, catalyzing the reaction at a rate close to the diffusion limit.
- Intermediate Formed: Through this reaction, all the carbon atoms from the initial glucose molecule are now in the form of Glyceraldehyde 3-phosphate (GAP). From one glucose molecule, we now have two molecules of GAP ready to enter the energy payoff phase.
- ATP Change: 0 ATP (No ATP is consumed or produced).
Purpose of the Interconversion:
This isomerization is crucial because:
- Ensures Efficient Pathway Progression: Only Glyceraldehyde 3-phosphate can move forward. By converting DHAP to GAP, the cell ensures that all carbon atoms from the original glucose are processed efficiently.
- Maintains Balance: The reaction is reversible, maintaining an equilibrium between DHAP and GAP, although the subsequent rapid consumption of GAP drives the equilibrium towards GAP formation.
End of Energy Investment Phase
This concludes the Energy Investment Phase (Reactions 1-5). We have now invested 2 ATP and converted one 6-carbon glucose into two 3-carbon Glyceraldehyde 3-phosphate molecules. The pathway is now ready to enter the Energy Payoff Phase.
The Energy Payoff Phase:
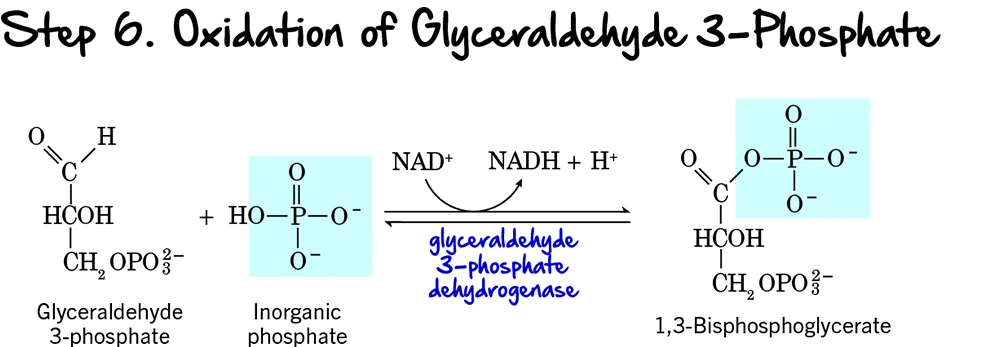
Step 6 (Oxidation and Phosphorylation of Glyceraldehyde 3-Phosphate)
We're now entering the Energy Payoff Phase of glycolysis! This is where the cell starts to recover its ATP investment and generate reducing power. Step 6 is the first reaction in this phase, and it's a crucial one as it involves both an oxidation event and the formation of a high-energy phosphate compound.
Reaction:
Each molecule of Glyceraldehyde 3-phosphate (GAP) undergoes a two-part transformation:
- Oxidation: The aldehyde group of GAP is oxidized to a carboxyl group.
- Phosphorylation: An inorganic phosphate (Pᵢ) group (not from ATP) is added to this newly formed carboxyl group, creating an acyl phosphate bond.
Key Features of Step 6:
- Enzyme: The enzyme catalyzing this reaction is Glyceraldehyde 3-phosphate Dehydrogenase.
- Intermediate Formed: 1,3-Bisphosphoglycerate (1,3-BPG).
- ATP Change: 0 ATP directly.
- NADH Production: +1 NADH is produced per molecule of GAP. Since each glucose yields two GAP molecules, this step generates a total of 2 NADH per glucose.
Purpose of this Step:
- Generation of Reducing Power (NADH): This is the only redox reaction in glycolysis. The electrons released during the oxidation of GAP are captured by NAD⁺, forming NADH. NADH is a crucial electron carrier that will later produce ATP in the electron transport chain (under aerobic conditions).
- Formation of a High-Energy Phosphate Bond: The newly formed bond at carbon 1 of 1,3-BPG is an acyl phosphate bond. This is a high-energy bond, meaning its hydrolysis releases significant free energy, which will be harnessed in the next step to synthesize ATP.
- Primer for ATP Synthesis: By creating 1,3-BPG with its high-energy phosphate, this step sets the stage for the first ATP generation in glycolysis via substrate-level phosphorylation.
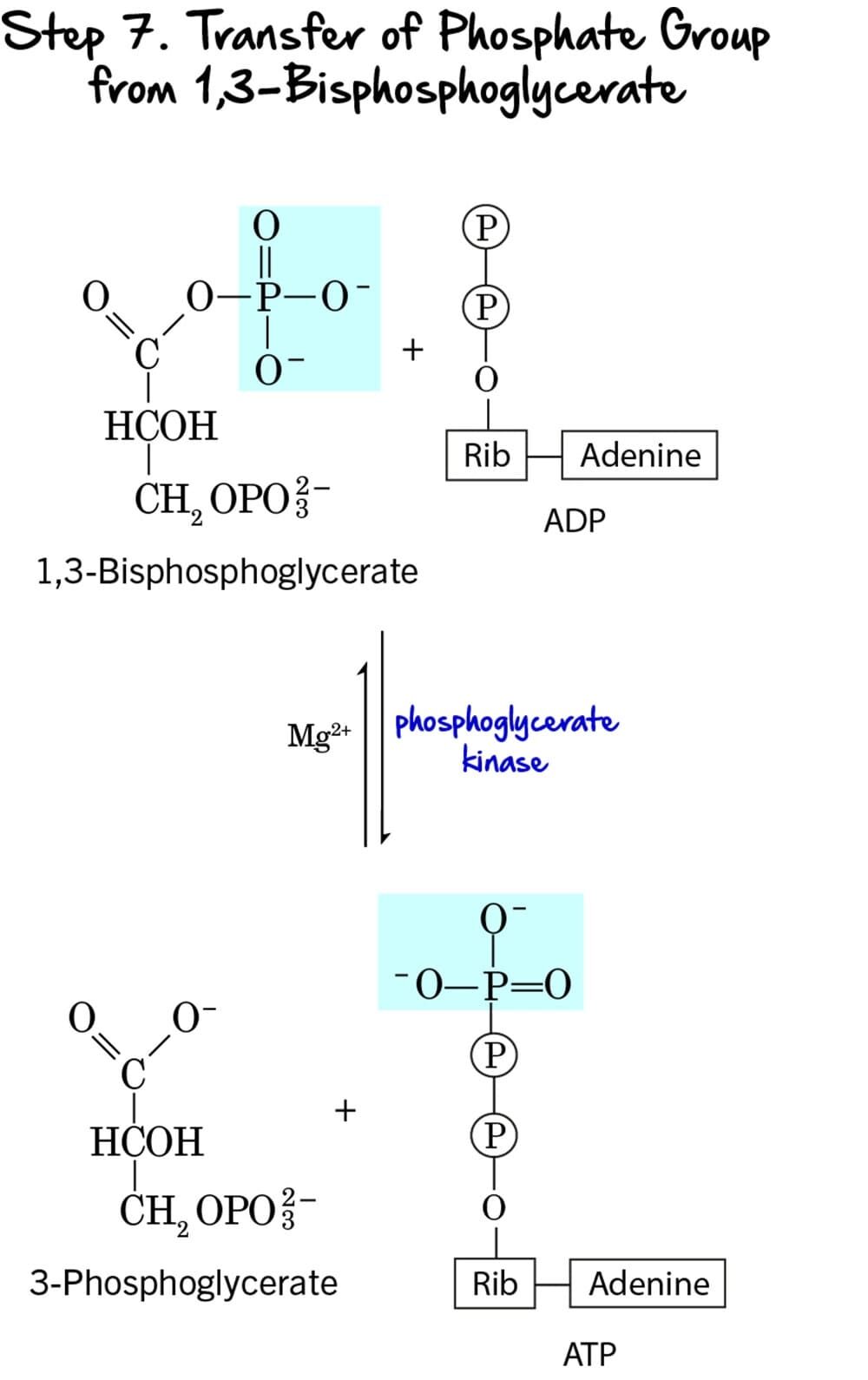
Step 7 - Substrate-Level Phosphorylation
This step marks the first direct production of ATP in glycolysis, utilizing the high-energy phosphate bond generated in the previous step.
Reaction:
The high-energy phosphate group from the C1 position of 1,3-Bisphosphoglycerate (1,3-BPG) is transferred to ADP, forming ATP. The remaining molecule is 3-Phosphoglycerate.
Key Features of Step 7:
-
Enzyme: The reaction is catalyzed by Phosphoglycerate Kinase. It requires
Mg²⁺as a cofactor. - Intermediate Formed: 3-Phosphoglycerate.
- ATP Change: +1 ATP is generated per molecule of 1,3-BPG. Since two molecules of 1,3-BPG are produced from each glucose, this step generates a total of 2 ATP per glucose.
- Mechanism: This is a classic example of substrate-level phosphorylation. ATP is formed directly from the transfer of a high-energy phosphate group from a substrate (1,3-BPG) to ADP.
Purpose of this Step:
- ATP Generation: This is the first actual ATP gain in glycolysis, partially recovering the energy invested in the preparatory phase.
- Energy Recovery: The energy released from the hydrolysis of the high-energy acyl phosphate bond in 1,3-BPG is efficiently captured to synthesize ATP.
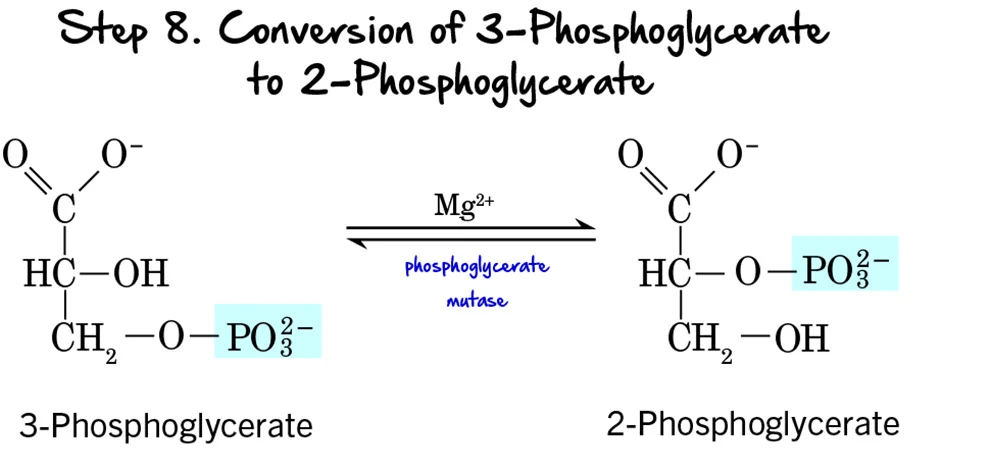
Step 8 - Migration of the Phosphate Group
After the first ATP-generating step, the molecule undergoes a structural rearrangement of its phosphate group to prepare for the next high-energy bond formation.
Reaction:
The phosphate group on 3-Phosphoglycerate moves from the carbon at position 3 to the carbon at position 2, forming 2-Phosphoglycerate. This is an intramolecular rearrangement.
Key Features of Step 8:
-
Enzyme: The reaction is catalyzed by Phosphoglycerate Mutase. Mutases are a class of isomerases that catalyze the transfer of a functional group within the same molecule. This enzyme requires
Mg²⁺as a cofactor. - Intermediate Formed: 2-Phosphoglycerate.
- ATP Change: 0 ATP (No ATP is consumed or produced).
Purpose of the Phosphate Migration:
This rearrangement is crucial for the subsequent steps:
- Positions for Dehydration: Moving the phosphate group to the C2 position places it in a strategic location to allow for the formation of a high-energy phosphate bond in the next step. It creates the necessary conditions for the dehydration reaction that follows.
- Increased Energy Potential: While 2-phosphoglycerate itself doesn't contain a high-energy bond, its structure is primed to become one through the elimination of water.
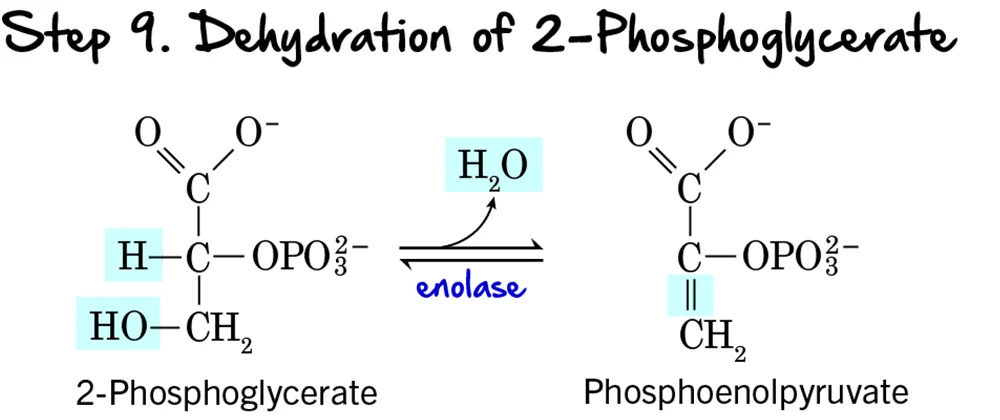
Step 9 - Dehydration of 2-Phosphoglycerate
Following the migration of the phosphate group, the molecule undergoes a dehydration reaction, which significantly raises the phosphoryl transfer potential of the phosphate group.
Reaction:
A molecule of water (H₂O) is removed from 2-Phosphoglycerate. This dehydration reaction creates a double bond within the molecule and forms the high-energy compound Phosphoenolpyruvate (PEP), which contains an "enol phosphate" bond.
Key Features of Step 9:
- Enzyme: The reaction is catalyzed by Enolase.
- Intermediate Formed: Phosphoenolpyruvate (PEP).
- ATP Change: 0 ATP (No ATP is consumed or produced).
Purpose of the Dehydration:
- Creation of a High-Energy Phosphate Bond: This is the most important outcome. The removal of water redistributes energy within the molecule, transforming a low-energy phosphate bond into a high-energy enol phosphate bond. The ΔG°' for the hydrolysis of PEP's phosphate bond is one of the highest in biochemistry.
- Preparation for ATP Synthesis: By forming PEP, the molecule is now poised to donate its phosphate group to ADP to generate ATP in the final step of glycolysis.
Clinical Relevance:
Enolase is inhibited by fluoride ions. This property is exploited in clinical settings: when blood samples are collected for glucose measurement, fluoride is often added to the collection tube to prevent glycolysis by red blood cells, ensuring the measured glucose concentration is accurate.
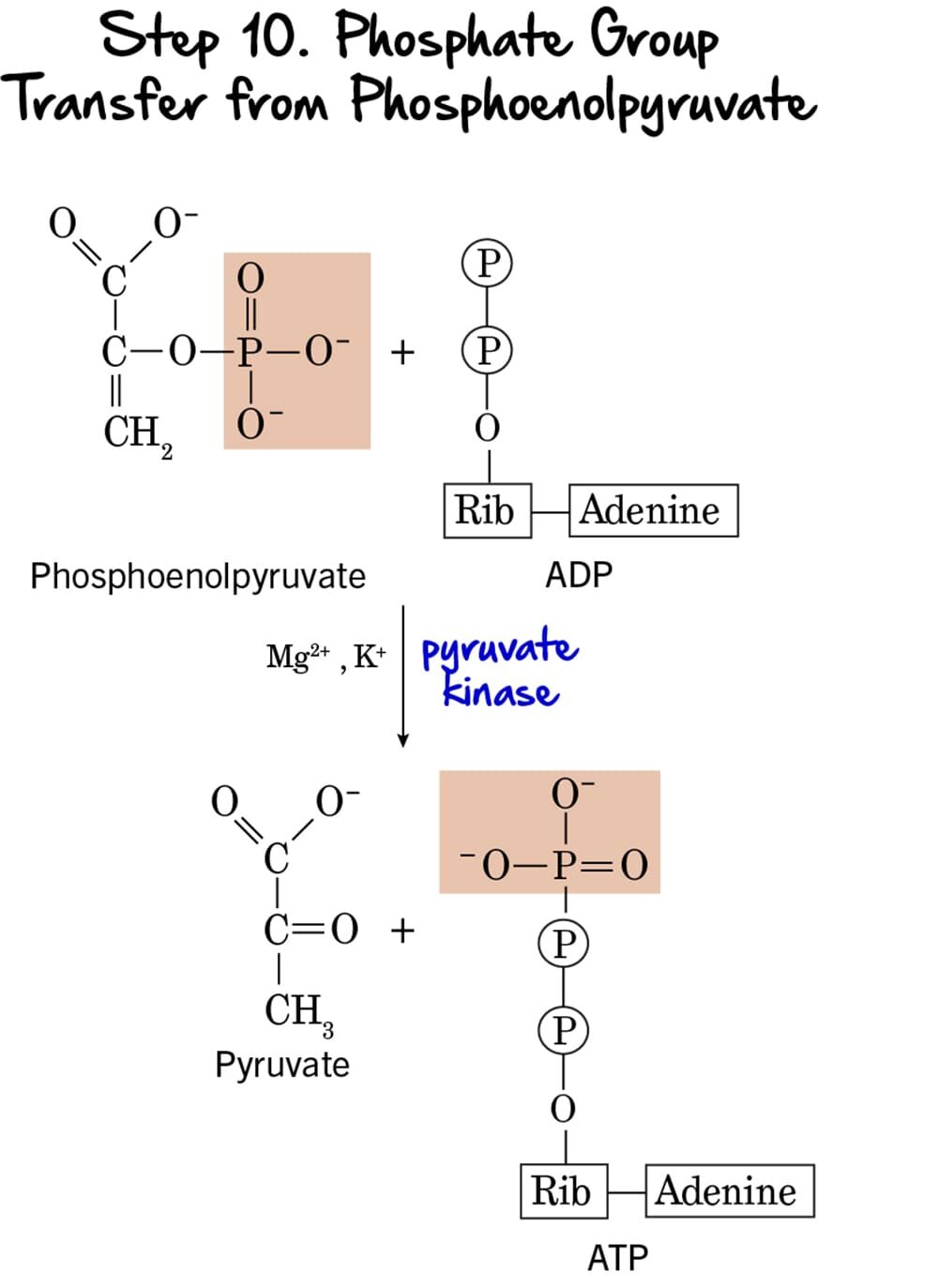
Step 10 - Phosphate Group Transfer from Phosphoenolpyruvate
This is the second and final ATP-generating step in glycolysis, again utilizing substrate-level phosphorylation to produce ATP and the ultimate end-product, pyruvate.
Reaction:
The high-energy phosphate group from Phosphoenolpyruvate (PEP) is transferred to ADP, yielding another molecule of ATP. The product remaining is Pyruvate. The initial enol form of pyruvate immediately tautomerizes to the more stable keto form.
Key Features of Step 10:
-
Enzyme: The reaction is catalyzed by Pyruvate Kinase, another key regulatory enzyme. It requires
Mg²⁺andK⁺as cofactors. - Intermediate Formed: Pyruvate.
- ATP Change: +1 ATP is generated per molecule of PEP. Since two molecules of PEP are produced from each glucose, this step generates a total of 2 ATP per glucose.
- Mechanism: This is the second instance of substrate-level phosphorylation in glycolysis.
Purpose of this Step:
- ATP Generation: This step provides the second net gain of ATP, completing the energy payoff.
- Formation of Pyruvate: Pyruvate is the end-product of glycolysis. Its fate depends on oxygen availability. Under aerobic conditions, it enters the mitochondria; under anaerobic conditions, it can be fermented.
- Irreversible Step & Regulation: This reaction is essentially irreversible, making Pyruvate Kinase a crucial regulatory enzyme.
Regulation of Pyruvate Kinase:
Pyruvate kinase is tightly regulated to control the flow of carbon through glycolysis:
-
Activation:
- Fructose 1,6-bisphosphate: This is a classic example of feed-forward activation. The product of PFK-1 (an earlier step) activates pyruvate kinase, ensuring intermediates are quickly processed.
-
Inhibition:
- High ATP: Signals abundant energy.
- Acetyl-CoA: An indicator of high energy status.
- Long-chain fatty acids: Another alternative fuel source.
Summary of Glycolysis (Net Reaction per glucose):
→
2 Pyruvate + 2 NADH + 2 H⁺ + 2 ATP
Glycolysis has now broken down one 6-carbon glucose molecule into two 3-carbon pyruvate molecules, produced a net of 2 ATP molecules, and generated 2 NADH molecules for further energy production.
Net Energy Yield of Glycolysis (per molecule of Glucose)
- ATP Consumed: 2 (Step 1 and Step 3)
- ATP Produced: 4 (Step 7 x 2, Step 10 x 2)
- Net ATP: 2 ATP
- NAD+ Reduced: 2 (Step 6 x 2)
- Net NADH: 2 NADH
This 2 net ATP and 2 NADH are the immediate energy harvest from glycolysis. The fate of pyruvate and NADH depends on the presence of oxygen.
Key Enzymes of Glycolysis: A Summary Table
| Step # | Enzyme Name | Reaction Catalyzed | Key Characteristics / Regulation |
|---|---|---|---|
| 1 | Hexokinase (I, II, III) | Glucose → G6P | Found in most tissues; High affinity for glucose; Inhibited by its product, G6P (feedback inhibition). Traps glucose in the cell. |
| 1 | Glucokinase (IV) | Glucose → G6P | Liver & pancreas; Low affinity (glucose sensor); Not inhibited by G6P; Induced by insulin. Important for glycogen/fat synthesis. |
| 3 | Phosphofructokinase-1 (PFK-1) | F6P → F1,6BP | RATE-LIMITING STEP. Activated by: High AMP, Fructose-2,6-bisphosphate. Inhibited by: High ATP, Citrate. |
| 10 | Pyruvate Kinase | PEP → Pyruvate | Activated by: Fructose-1,6-bisphosphate (feed-forward). Inhibited by: High ATP, Acetyl-CoA, fatty acids. Deficiency can cause hemolytic anemia. |
Additional Notes on Isoenzymes:
Isoenzymes (or isozymes) are different forms of an enzyme that catalyze the same reaction but are encoded by different genes, allowing for fine-tuning of metabolic control in different organs.
- Hexokinase vs. Glucokinase: Glucokinase's low affinity in the liver means it only works significantly when glucose is abundant, allowing other tissues (like the brain) to get glucose first when supplies are limited.
- Pyruvate Kinase L-type (Liver) vs. M-type (Muscle): The liver (L-type) form can be inhibited by glucagon (a hormone signaling low blood sugar) to conserve glucose. The muscle (M-type) form is not, as muscles prioritize their own energy supply.
Differentiating Between Aerobic and Anaerobic Glycolysis
The primary challenge after glycolysis is to regenerate NAD⁺ from NADH. If NAD⁺ is not regenerated, glycolysis will stop.
1. Anaerobic Glycolysis (No Oxygen Present)
When oxygen is scarce (e.g., in vigorously contracting muscles or red blood cells), cells convert pyruvate to lactate to regenerate NAD⁺.
- Reaction: Pyruvate is reduced to lactate.
- Enzyme: Lactate Dehydrogenase (LDH)
- Key Process: In this reaction, NADH is oxidized back to NAD⁺.
Pyruvate + NADH + H⁺ → Lactate + NAD⁺ - Net Products (per glucose): 2 ATP, 2 Lactate
- Physiological Significance:
- Allows rapid, short-term ATP production, vital for tissues like red blood cells (no mitochondria) and muscles during intense exercise.
- Cori Cycle: Lactate from muscle goes to the liver, is converted back to glucose (gluconeogenesis), and returned to the muscle.
- Limited efficiency; lactate accumulation can lead to fatigue.
2. Aerobic Glycolysis (Oxygen Present)
When oxygen is abundant, pyruvate and NADH are further oxidized in the mitochondria to generate much more ATP.
-
Fate of Pyruvate:
- Pyruvate is transported into the mitochondrial matrix.
- It is converted to Acetyl-CoA by the Pyruvate Dehydrogenase Complex (PDC).
- Acetyl-CoA then enters the Tricarboxylic Acid (TCA) Cycle.
-
Fate of NADH:
- Cytoplasmic NADH cannot directly enter the mitochondria. Its electrons are transferred via shuttle systems:
- Malate-Aspartate Shuttle (heart, liver): More efficient, yields mitochondrial NADH.
- Glycerol-3-Phosphate Shuttle (muscle, brain): Less efficient, yields mitochondrial FADH₂.
- The mitochondrial NADH and FADH₂ then donate their electrons to the ETC for Oxidative Phosphorylation.
- Cytoplasmic NADH cannot directly enter the mitochondria. Its electrons are transferred via shuttle systems:
Overall ATP Yield (Aerobic vs. Anaerobic):
- Anaerobic Glycolysis: Net 2 ATP per glucose.
- Aerobic Glycolysis (and subsequent oxidation): Approximately 30-32 ATP per glucose.
Summary of Pyruvate Fates:
- Anaerobic Conditions: Pyruvate → Lactate (to regenerate NAD⁺).
- Aerobic Conditions: Pyruvate→ Acetyl-CoA → TCA Cycle (for complete oxidation and much more ATP).
Regulation of Glycolysis
The body doesn't just run metabolic pathways at full throttle. A sophisticated system of regulation ensures that glucose is utilized efficiently and ATP is produced only when needed. Glycolysis is primarily regulated at three irreversible steps, each catalyzed by a key enzyme:
- Hexokinase / Glucokinase (Step 1)
- Phosphofructokinase-1 (PFK-1) (Step 3)
- Pyruvate Kinase (Step 10)
These enzymes act as "gatekeepers" that can be turned up or down through two main mechanisms:
1. Allosteric Control (Immediate, Short-Term Regulation)
Allosteric regulation involves molecules binding to an enzyme at a site other than the active site, causing a conformational change that either increases (activator) or decreases (inhibitor) its activity. This provides rapid feedback based on the cell's immediate energy status.
Hexokinase (Step 1)
Inhibited by:
- Glucose-6-phosphate (its own product, providing feedback inhibition).
PFK-1 (Step 3)
(Rate-Limiting Step)
Activated by:
- AMP & ADP (signals low energy).
- Fructose-2,6-bisphosphate (signals high glucose).
Inhibited by:
- ATP (signals high energy).
- Citrate (signals TCA cycle is full).
Pyruvate Kinase (Step 10)
Activated by:
- Fructose-1,6-bisphosphate (feed-forward activation).
Inhibited by:
- ATP, Acetyl-CoA, Fatty Acids (all signal high energy).
- Alanine.
2. Hormonal Control (Longer-Term, Systemic Regulation)
Hormones, primarily insulin and glucagon, regulate glycolysis (especially in the liver) to maintain whole-body blood glucose homeostasis. They achieve this mainly by changing the amount or activity of key enzymes.
Insulin (High Blood Glucose)
Promotes Glycolysis:
- Increases the synthesis (gene expression) of glucokinase, PFK-1, and pyruvate kinase in the liver.
- Activates PFK-2, which produces Fructose-2,6-bisphosphate, a powerful activator of PFK-1.
Glucagon (Low Blood Glucose)
Inhibits Glycolysis (in the Liver):
- Decreases the synthesis of key glycolytic enzymes.
- Inactivates Pyruvate Kinase via phosphorylation, redirecting intermediates towards making new glucose (gluconeogenesis).
- Inactivates PFK-2, reducing levels of the PFK-1 activator Fructose-2,6-bisphosphate.
3. Gene Expression (Long-Term Adaptation)
The rates of synthesis of glycolytic enzymes can also be regulated at the level of gene transcription. For instance, in conditions of chronic high glucose or in some cancers, the expression of glycolytic enzymes can be upregulated.
Clinical Relevance of Glycolysis
Understanding glycolysis is not just an academic exercise; it's essential for comprehending the pathophysiology of numerous diseases and for developing therapeutic strategies.
1. Cancer (The Warburg Effect)
Many cancer cells exhibit significantly increased rates of glycolysis, even with sufficient oxygen ("Warburg Effect").
Why? While less efficient, rapid glycolysis provides ATP and metabolic intermediates needed for fast proliferation. In low-oxygen tumors, HIF-1 boosts glycolytic enzymes.
Clinical Application: PET Scans use a radioactive glucose analog (FDG) that is avidly taken up by cancer cells, making them "light up" on the scan to locate tumors.
2. Red Blood Cell Metabolism
Mature red blood cells (RBCs) lack mitochondria and are entirely dependent on anaerobic glycolysis for ATP.
ATP in RBCs is used to:
- Maintain ion gradients (Na⁺/K⁺ pump).
- Preserve the biconcave shape.
A side-product, 2,3-BPG, is crucial as it binds to hemoglobin and facilitates oxygen release to tissues.
3. Pyruvate Kinase Deficiency
A genetic defect in the pyruvate kinase enzyme, primarily affecting RBCs.
Consequences: Insufficient ATP production in RBCs leads to failure of ion pumps, cell swelling, and premature destruction (hemolysis).
Clinical Presentation: Chronic hemolytic anemia, characterized by fatigue, jaundice, and an enlarged spleen.
4. Lactic Acidosis
A metabolic condition with an accumulation of lactate in the blood, leading to a decrease in blood pH.
Causes:
- Tissue Hypoxia (Type A): Most common; caused by shock, severe anemia, or intense exercise. Tissues switch to anaerobic glycolysis, overproducing lactate.
- Mitochondrial Dysfunction (Type B): Defects in the ETC cause pyruvate to be shunted to lactate.
It is a serious condition that can lead to organ dysfunction.
5. Diabetes Mellitus (Indirect Relevance)
While not a direct defect in glycolysis, its regulation is profoundly affected in diabetes.
- Insulin Resistance (Type 2): Cells become less responsive to insulin, which impairs glucose uptake and reduces the stimulation of glycolytic enzymes, leading to higher blood glucose.
- Insulin Deficiency (Type 1): Lack of insulin means glucose cannot be efficiently taken up by many tissues, and liver glycolysis is not stimulated, contributing to hyperglycemia.
Biochemistry: Glycolysis Exam
Test your knowledge with these 40 questions.
Glycolysis Exam
Question 1/40
Exam Complete!
Here are your results, .
Your Score
38/40
95%