Biochemistry Intro : Bonding & Water
Learning Objectives
- Chemical Bonds:
- Covalent bonds: Sharing electrons (the most important type in biochemistry).
- Ionic bonds: Transferring electrons (less common in biological molecules).
- Hydrogen bonds: Weaker bonds, but SUPER important for water and protein structure.
- Water:
- Its unique properties: Polarity, hydrogen bonding.
- Why it's essential for life: How it dissolves things, its role in temperature regulation.
Introduction to Chemical Bonds
We've covered atoms, the fundamental building blocks. However, atoms rarely exist in isolation. The connection that holds atoms together is known as a chemical bond.
Chemical bonds are the powerful attractive forces that hold atoms together to form molecules and compounds. They are entirely about the behavior and interactions of an atom's outermost electrons, called valence electrons. The primary reason atoms form bonds is to achieve a more stable state, typically by having a full outer electron shell.
Chemical Bonds
We've explored atoms, the fundamental building blocks. However, in nature, atoms rarely exist in isolation. They are almost universally connected to other atoms. This crucial connection is known as a chemical bond.
Definition: Chemical bonds are the powerful attractive forces that hold atoms together to form molecules and compounds. They are, in essence, the "glue" of chemistry, responsible for creating all the complex structures we see, from simple water molecules to intricate proteins and DNA.
- The Role of Electrons: Chemical bonds are not made of literal glue; rather, they are entirely about the behavior and interactions of an atom's electrons, particularly its outermost electrons, which are called valence electrons.
- The Drive for Stability: The primary reason atoms form bonds is to achieve a more stable, "happy" state. For most atoms, this is attained by having a full outer electron shell, often with eight electrons (the "octet rule"). Atoms will gain, lose, or share valence electrons to reach this desired configuration.
There are two main types of strong chemical bonds that are particularly important in biochemistry and healthcare: Covalent Bonds and Ionic Bonds.
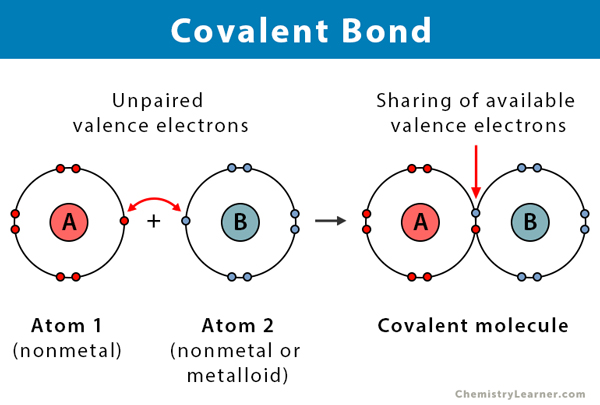
I. Covalent Bonds: The "Sharing is Caring" Bond
A covalent bond forms when two atoms share one or more pairs of electrons. It's a true partnership, and these are the strong bonds that form the backbone of the vast majority of molecules in our bodies.
- Mechanism: Each atom contributes one electron to the shared pair, and this shared pair is then mutually attracted to the nuclei of both atoms, effectively holding them together.
- Prevalence: Covalent bonds are the most common type of bond found in organic molecules – the fundamental molecules of life, including carbohydrates, lipids, proteins, and nucleic acids.
- Valence Electrons Revisited: Remember, valence electrons are the electrons in the outermost shell of an atom. They are the key players in chemical bonding and are the ones involved in the electron-sharing of covalent bonds.
Analogy for Covalent Bonding
Imagine two people who both deeply desire to own a dog, but neither can financially manage the full cost and responsibility alone. They decide to co-own one. They are now "bonded" together by their shared pet. The shared dog represents the shared pair of electrons, and the arrangement benefits both owners. The dog effectively belongs to both, much like the shared electrons are attracted to both atomic nuclei.
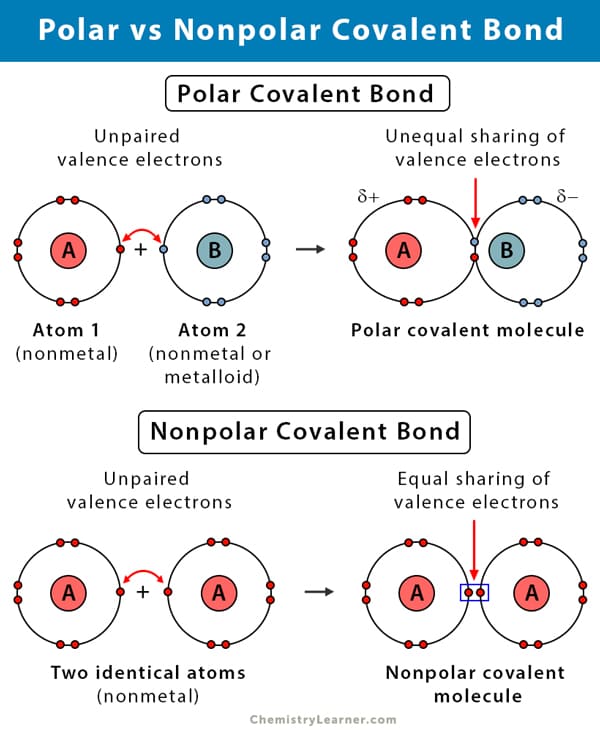
Types of Covalent Bonds: Not All Sharing is Equal
Even within covalent bonds, the sharing of electrons isn't always perfectly equitable. This leads to two important subtypes:
a) Nonpolar Covalent Bonds: Equal Sharing
Definition: Nonpolar covalent bonds occur when electrons are shared equally between two atoms.
Electronegativity Connection: This equal sharing happens when the two atoms have very similar electronegativity. Electronegativity is a measure of an atom's intrinsic ability to attract shared electrons. If their "pull" is roughly equivalent, the electrons spend an equal amount of time around each nucleus.
Result: There is no significant charge difference across the bond. The molecule remains electrically symmetrical.
Examples:
- O=O (oxygen gas): Two identical oxygen atoms share electrons perfectly.
- C-C bonds: Carbon atoms share electrons equally.
- C-H bonds: Carbon and hydrogen have similar electronegativities, forming nearly nonpolar bonds found in fats and oils.
Analogy: If two identical twins co-own a dog, the dog will spend exactly half its time at each house. The sharing is perfectly equal.
b) Polar Covalent Bonds: Unequal Sharing
Definition: Polar covalent bonds occur when electrons are shared unequally between two atoms.
Electronegativity Difference: This arises when one atom is significantly more electronegative than the other, exerting a stronger "pull" on the shared electrons.
Result: This creates a slight partial negative charge (δ−) on the more electronegative atom and a slight partial positive charge (δ+) on the less electronegative one. The molecule has distinct "poles" of charge.
Examples:
- O-H bonds: Oxygen is much more electronegative than hydrogen.
- N-H bonds: Nitrogen is also more electronegative than hydrogen.
Crucial for Water (H₂O): The highly electronegative oxygen pulls electrons from the hydrogen atoms, leaving the oxygen end partially negative (δ−) and the hydrogen ends partially positive (δ+). This polarity is essential for all life.
Analogy: If a big, strong adult co-owns a dog with a small child, the dog will spend more time at the adult's house. The sharing is unequal, creating a partial imbalance.
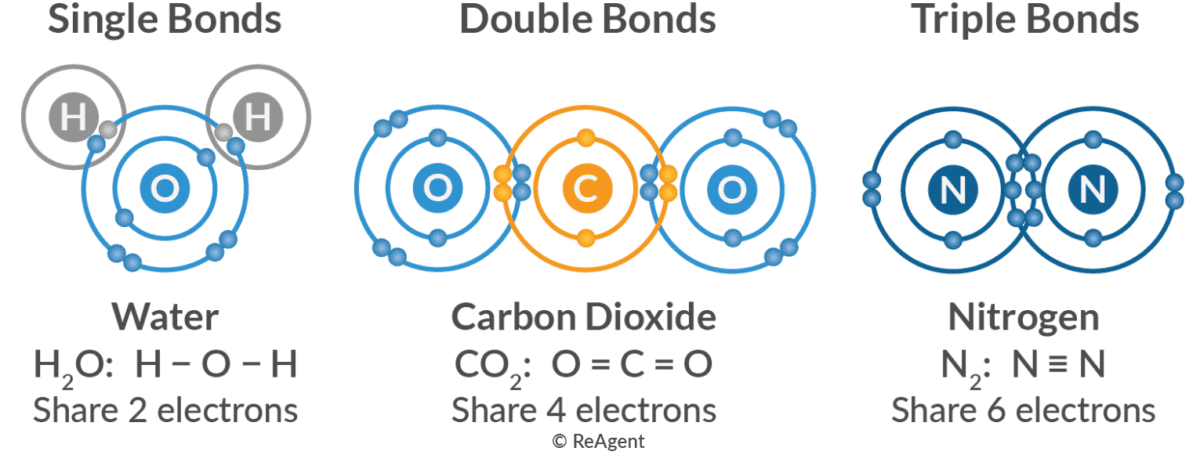
Single, Double, and Triple Covalent Bonds: Degrees of Sharing
Covalent bonds can also vary in the number of electron pairs shared:
- Single Bond: Atoms share one pair of electrons (2 electrons total). These bonds are generally flexible and allow rotation.
- Double Bond: Atoms share two pairs of electrons (4 electrons total). These are stronger and shorter and restrict rotation.
- Triple Bond: Atoms share three pairs of electrons (6 electrons total). These are the strongest and shortest and entirely prevent rotation.
Why Understanding Covalent Bond Polarity is Vital in Nursing and Biology:
The distinction between nonpolar and polar covalent bonds profoundly impacts biological systems:
"Like Dissolves Like": The Basis of Solutions:
- Why IV Fluids Work: Polar water readily dissolves charged ions (like Na⁺ and Cl⁻ from salt), which explains why saline solution is the basis of most IV fluids.
- Why Oil and Water Don't Mix: Nonpolar substances (oils, fats) do not dissolve in polar water. This rule is fundamental to many biological processes.
Cell Membrane Function:
- Permeability: Small, nonpolar molecules (like O₂, CO₂) can pass directly through the nonpolar lipid bilayer of the cell membrane.
- Selective Transport: Polar molecules (water, glucose) and charged ions (Na⁺, K⁺) are repelled by the membrane and require specialized protein channels to cross.
Protein and DNA Structure and Function:
The intricate 3D shapes of proteins and DNA are maintained by attractions between the partial positive (δ⁺) and negative (δ⁻) regions of these giant molecules. A disruption in this balance (e.g., from changes in pH or temperature) can cause them to unfold (denature) and lose their function.
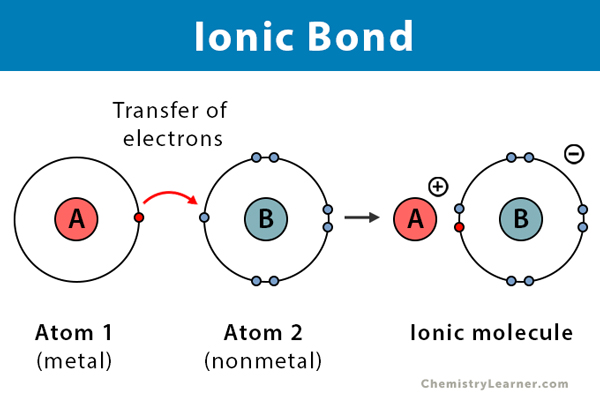
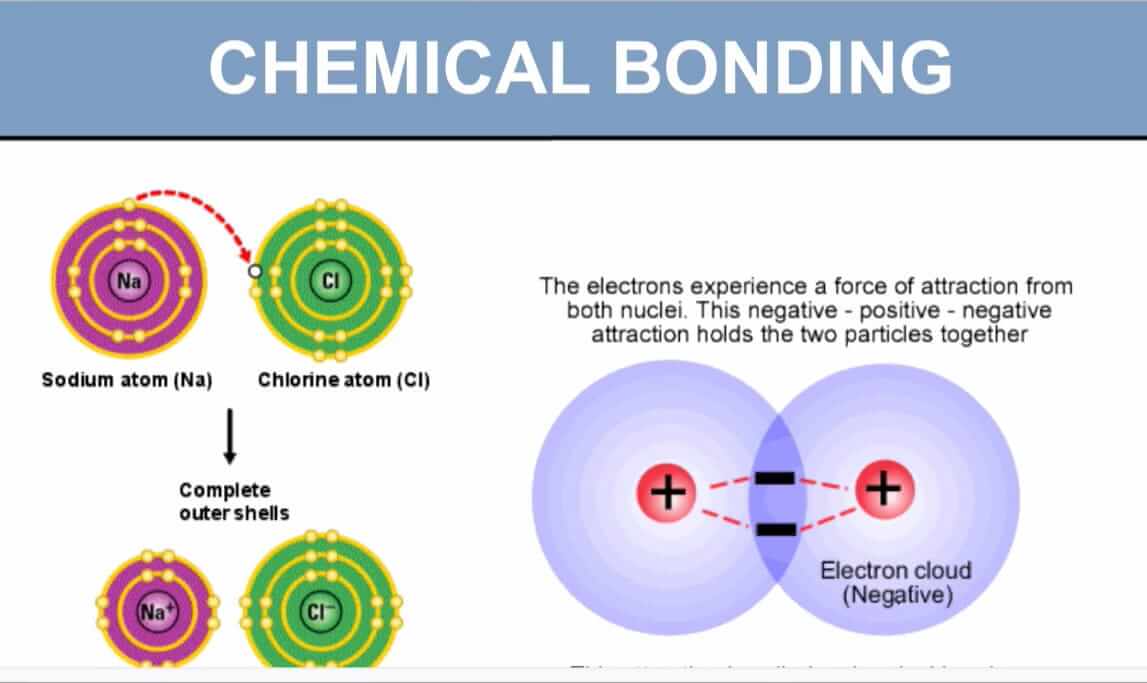
II. Ionic Bonds: The "Give and Take" Bond
While covalent bonds involve sharing electrons, ionic bonds represent a more dramatic interaction: the complete transfer of one or more electrons from one atom to another.
Mechanism:
- One atom (usually a metal) readily loses electrons to achieve a stable outer shell, transforming into a positively charged ion called a cation.
- Another atom (usually a non-metal) readily gains those electrons to achieve its own stable outer shell, becoming a negatively charged ion called an anion.
- The resulting oppositely charged ions are then powerfully attracted to each other by strong electrostatic forces. This attraction is the ionic bond.
A large difference in electronegativity between the two atoms is what drives this electron transfer.
Analogy for Ionic Bonding
Instead of co-owning, imagine one person gives their dog to another. The first person feels lighter and happier (like a cation, having lost something). The second person is also happy and stable (like an anion, having gained something). These two are now strongly connected through this exchange.
Example: Sodium Chloride (NaCl - Common Table Salt)
- Sodium (Na) has 1 electron in its outer shell. It easily loses this electron to become a positively charged Na⁺ cation.
- Chlorine (Cl) has 7 electrons in its outer shell. It easily gains one electron to become a negatively charged Cl⁻ anion.
- The Bond: The strong electrostatic attraction between the positive Na⁺ ion and the negative Cl⁻ ion forms the ionic bond.
Why Ionic Bonds are Important in Biology and Nursing
- Electrolytes: Many essential electrolytes in the body (e.g., Na⁺, K⁺, Ca²⁺, Cl⁻) are ions crucial for nerve transmission, muscle contraction, and fluid balance.
- Mineral Components: Bones and teeth are largely composed of ionic compounds like calcium phosphate.
- Drug Action: Many medications are ionic compounds or interact with ions in the body.
Covalent vs. Ionic Bonds: A Comparison
| Feature | Covalent Bond | Ionic Bond |
|---|---|---|
| Electron Action | Shared between atoms | Transferred from one atom to another |
| Result | Formation of molecules | Formation of ions (cations and anions) |
| Charge | No overall charge (nonpolar) or partial charges (polar) | Full positive and negative charges on ions |
| Strength | Strong | Strong (especially in solid crystals) |
| Key Player | Small difference in electronegativity | Large difference in electronegativity |
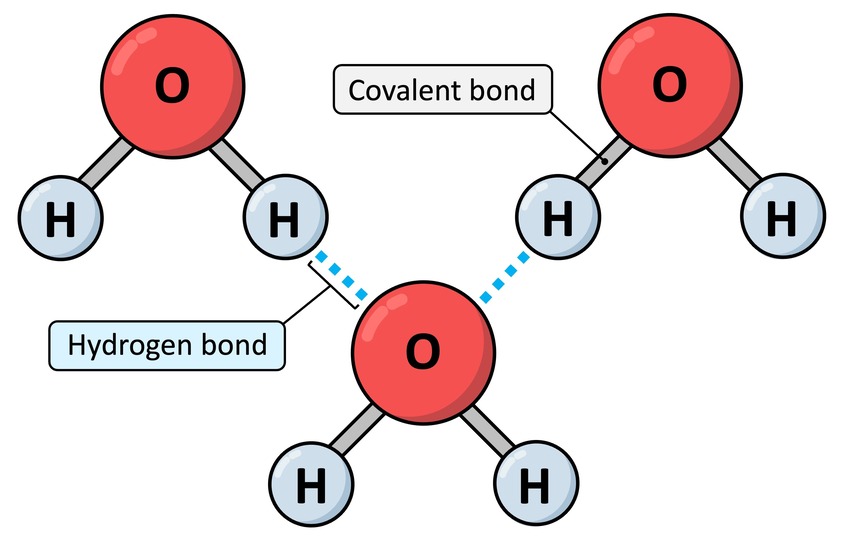
III. Hydrogen Bonds
After strong intra-molecular bonds (like covalent and ionic), we now turn to a weaker but incredibly important inter-molecular force: Hydrogen Bonds. These are vital for the structure of water, proteins, and DNA.
Definition: A hydrogen bond is a relatively weak attractive force that forms between a partially positive hydrogen atom (δ+) and a partially negative atom (δ-, usually O or N) on an adjacent molecule or part of a molecule.
While individually weak (5-10% of a covalent bond's strength), the cumulative effect of many hydrogen bonds can create a very significant and stable overall force, like Velcro.
Analogy for Hydrogen Bonds
Imagine a person with a very friendly (partially positive) dog (the hydrogen atom). This dog loves to briefly greet other reserved dogs (the partially negative O or N atoms) in the park. Each greeting is a temporary, gentle interaction. But if there are many such friendly dogs, all these brief greetings collectively create a bustling, interconnected social scene.
Why Hydrogen Bonds Are Life-Giving (and Crucial in Nursing)
Hydrogen bonds are the primary reason for many of life's essential chemical properties:
- Water's Unique Properties: They give water its high boiling point, high specific heat (regulating body temperature), cohesion (surface tension), and adhesion. Without them, water would be a gas at room temperature.
- Protein Structure: They are critical for stabilizing the complex 3D shapes of proteins. Maintaining these shapes is essential for protein function.
- DNA Structure: They hold the two complementary strands of the DNA double helix together. Their ability to easily "unzip" and "re-zip" is fundamental to DNA replication and gene expression.
- Drug-Receptor Interactions: Many drugs exert their effects by forming hydrogen bonds with specific receptor sites on proteins or nucleic acids.
Water - The Solvent of Life: H₂O
Every single chemical reaction vital for life, from the intricate metabolic pathways that process your last meal to the complex electrochemical signals enabling thought, occurs in an aqueous (water-based) environment. It's no coincidence that water constitutes approximately 60-70% of the human body by weight. Its truly unique and extraordinary properties create the perfect conditions for the chemistry of life to unfold.
What Makes Water So Special? The Power of Polarity and Hydrogen Bonds
The answer to water's exceptional nature lies directly in its molecular structure and, crucially, its unparalleled ability to form hydrogen bonds.
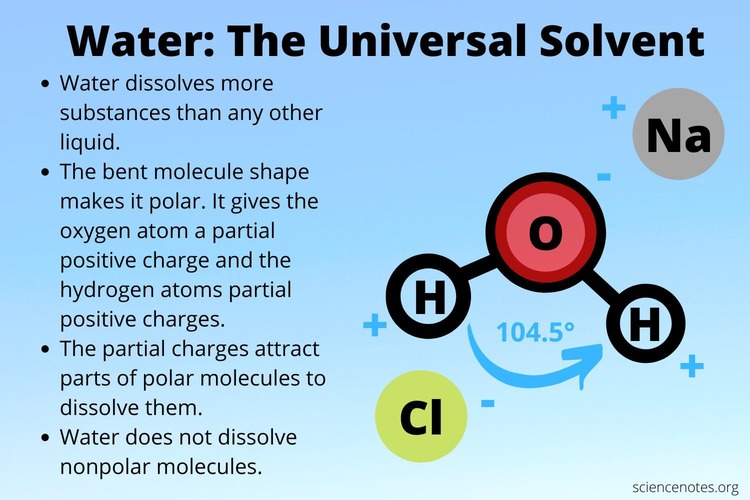
Let's quickly revisit the structure of a water molecule (H₂O):
- Bent Shape: The water molecule adopts a bent, V-shaped or angular geometry. This non-linear arrangement is critical because it ensures the partial positive charges (on the hydrogens) are distinct from the partial negative charges (on the oxygen).
- Electronegativity Difference: The Oxygen atom is an "electron hog" – it is highly electronegative.
- Unequal Sharing: In the two O-H covalent bonds, oxygen pulls the shared electrons significantly closer to itself than the hydrogen atoms do.
- Partial Charges: This unequal sharing creates a partial negative charge (δ⁻) on the Oxygen atom and partial positive charges (δ⁺) on each of the two Hydrogen atoms.
Because of this separation of charge, a single water molecule acts like a tiny, permanent electrical dipole – essentially, a miniature magnet. This inherent polarity is the foundation for everything that makes water so vital.
Hydrogen Bonding
When you observe a glass of water, it's not merely a collection of isolated H₂O molecules floating aimlessly. Instead, they are actively and dynamically "sticking" to one another through a continuous network of hydrogen bonds. The slightly positive (δ⁺) Hydrogen of one water molecule is electrostatically attracted to the slightly negative (δ⁻) Oxygen of a neighboring water molecule. Each water molecule can form up to four hydrogen bonds with its neighbors, creating a highly interconnected fluid.

Consequences of Hydrogen Bonds
The pervasive network of hydrogen bonds among water molecules gives rise to its extraordinary properties:
Cohesion & Surface Tension
Cohesion: The strong mutual attraction between water molecules means they "stick together." This is why water droplets form.
Surface Tension: At the air-water interface, water molecules are more attracted to each other than to the air, creating a "skin-like" effect.
Capillary Action: The combined effect of cohesion and adhesion allows water to move upwards against gravity in narrow tubes, critical for transport in plants and biological vessels.
Adhesion
Water molecules, being polar, are attracted to other polar or charged surfaces. This is why water "wets" materials like glass and adheres to the internal surfaces of blood vessels.
High Specific Heat Capacity
Definition: Water takes a large amount of thermal energy to raise its temperature.
Mechanism: A significant portion of incoming heat must first be used to break the extensive network of hydrogen bonds.
Biological Importance: This is vital for temperature regulation. Your body, being mostly water, can absorb substantial heat with only small fluctuations in core body temperature.
High Heat of Vaporization
Definition: A large amount of energy is required to change water from a liquid to a gas.
Mechanism: Energy must be supplied to break the hydrogen bonds before molecules can escape as vapor.
Biological Importance: This underlies the cooling effect of sweating. As water evaporates from the skin, it carries away a large amount of heat.
Less Dense as a Solid (Ice Floats)
Unusual Property: Solid water (ice) is less dense than liquid water.
Mechanism: At freezing, hydrogen bonds fix water molecules into a rigid lattice where they are spaced further apart than in liquid form.
Biological Importance: Floating ice forms an insulating layer on bodies of water, protecting aquatic life below from freezing.
Water as the "Universal Solvent"
This is arguably the most important property for biochemistry. Because water molecules are tiny, polar "magnets," they are incredibly adept at pulling apart and surrounding other charged (ionic) or polar molecules.
1. Hydrophilic ("Water-Loving")
These are polar or charged molecules that readily dissolve in water.
Example 1: Salt (NaCl):
When salt is added to water, the partially negative Oxygen ends of water molecules surround the positive Na⁺ ions, and the partially positive Hydrogen ends surround the negative Cl⁻ ions. This process, called hydration, pulls the salt crystal apart.
Example 2: Sugar (Glucose, C₆H₁₂O₆):
Glucose is a polar molecule with numerous O-H bonds. Water molecules are attracted to these partial charges, surrounding the glucose molecule and pulling it into solution.
2. Hydrophobic ("Water-Fearing")
These are non-polar molecules that do not dissolve in water because they lack charges for water to interact with.
Example: Oil & Fat:
Oils and fats are non-polar hydrocarbons. Water molecules are more strongly attracted to each other than to the oil, so they exclude the oil molecules, forcing them to clump together. This is the hydrophobic effect, a critical driving force for structures like cell membranes.
Why Health Workers MUST Understand Water's Properties: Clinical Relevance
These concepts explain fundamental aspects of human physiology, disease processes, and the efficacy of medications.
Blood Transport:
- Your blood plasma is approximately 92% water.
- Hydrophilic substances like glucose and sodium ions dissolve directly in blood plasma.
- Hydrophobic substances like cholesterol and fats must be packaged inside transport proteins called lipoproteins (e.g., LDL, HDL) to be carried through the bloodstream.
Drug Action & Delivery:
- Intravenous (IV) medications must be hydrophilic enough to dissolve in blood plasma.
- A drug that needs to cross the predominantly hydrophobic cell membrane must possess a degree of hydrophobic character to traverse the lipid bilayer.
Cell Membrane Structure and Function:
The entire framework of the cell membrane is a lipid bilayer, where hydrophilic "heads" face the watery environments, and hydrophobic "tails" tuck into the interior. This structure creates a selective barrier that dictates which molecules can pass through unaided versus those that require protein channels.
Protein Folding and Function:
In the watery environment of the cell, proteins spontaneously fold so their hydrophobic parts are sequestered in the core, while hydrophilic parts remain on the surface. Misfolding of proteins due to errors in this balance underlies many diseases like Alzheimer's and Parkinson's.
Enzyme Activity:
Enzymes rely on specific 3D structures to function. The hydrophilic and hydrophobic interactions within the active site of an enzyme dictate its specificity and efficiency.
Biochemistry Lesson Two
Chemical Bonds & Atomic Structure
Test your knowledge with these 20 questions.
Biochemistry: Bonds & Structure
Question 1/20
Quiz Complete!
Here are your results, .
Your Score
18/20
90%