Body Fluids: And Compartments
Body Fluids
To truly appreciate the dynamics of body fluids, we first need to understand where all this fluid is located within the body. Imagine your body as a system of interconnected containers, each holding a specific type of fluid. These "containers" are what we call body fluid compartments.
The human body is largely composed of water, and this water isn't just free-flowing; it's meticulously organized into various functional compartments. This compartmentalization is key to maintaining cellular and systemic homeostasis.
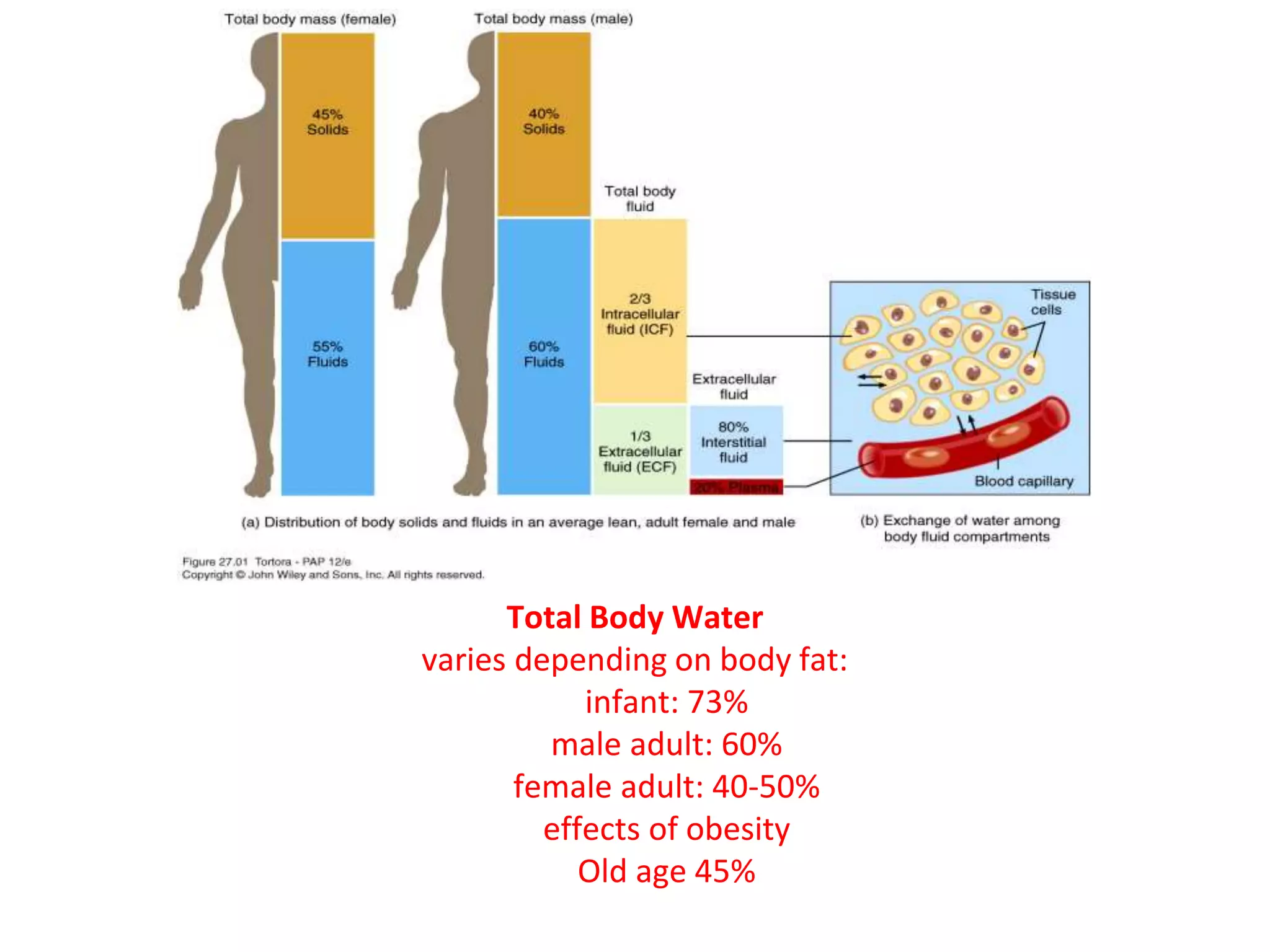
1. Total Body Water (TBW)
TBW refers to all the water contained within the body. It represents a significant proportion of body mass.
Proportion:
Approximately 60% of an adult's body weight is water. This percentage can vary significantly based on several factors:
- Age: Infants (up to 75-80%), Adults (~60%), and the Elderly (can drop to 45-50%).
- Sex: Females generally have a slightly lower TBW percentage than males because they typically have a higher percentage of adipose tissue (fat), which contains very little water.
- Body Fat Content: Individuals with higher body fat percentages will have lower TBW percentages, and vice-versa.
Composition of Water:
TBW is not pure water; it contains numerous dissolved solutes, including electrolytes, proteins, nutrients, gases, and waste products. The total amount of water in an adult human body constitutes about 50-70% of the total body weight. This water is not uniformly distributed but is divided into two primary compartments, which are further subdivided:
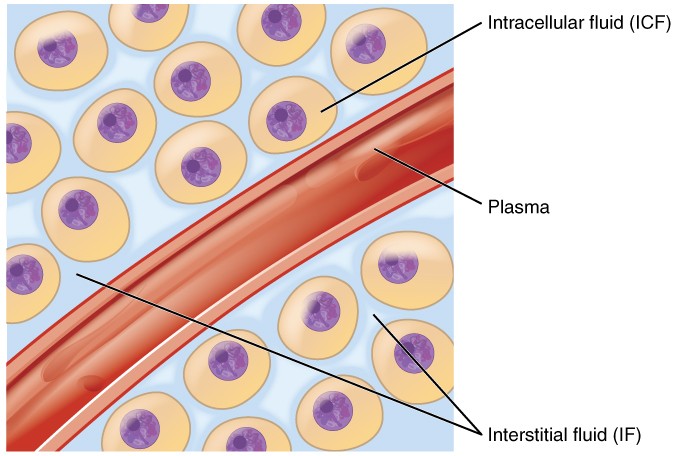
A. Intracellular Fluid (ICF)
Location: The ICF is the fluid found within the cells of the body. It is the immediate environment where the vast majority of cellular metabolic activities take place.
Proportion and Significance: The ICF constitutes the largest single fluid compartment, accounting for approximately two-thirds (2/3) of the Total Body Water (TBW). In an adult male weighing 70 kg, this would be roughly 28 liters (40% of body weight). This large volume underscores its critical role: it directly bathes the cellular machinery, providing the aqueous medium for all intracellular biochemical reactions.
Composition - The Cell's Internal Environment:
- Major Cations:
- Potassium (K⁺): The predominant cation in the ICF. Its high concentration is crucial for nerve impulse transmission, muscle contraction, and maintaining cell volume.
- Magnesium (Mg²⁺): Vital as a cofactor for numerous enzymatic reactions, particularly those involving ATP.
- Major Anions:
- Phosphate (PO₄³⁻): A critical component of energy currency (ATP), nucleic acids, and intracellular buffering systems.
- Proteins: The ICF is rich in large, negatively charged protein molecules that contribute to osmolarity and act as important buffers.
- Low Concentrations: In stark contrast to the ECF, Sodium (Na⁺) and Chloride (Cl⁻) concentrations are very low within the ICF.
Key Characteristics - Functional Blueprint:
- Selective Permeability of the Cell Membrane: The plasma membrane is the critical barrier separating the ICF from the ECF, maintaining the distinct chemical composition of the ICF.
- Metabolic Engine: The ICF houses the cell's entire metabolic machinery – organelles like mitochondria, ribosomes, and the nucleus.
- Osmotic Equilibrium: Despite vastly different chemical compositions, the total osmotic concentration (osmolarity) of the ICF is normally in dynamic equilibrium with the ECF.
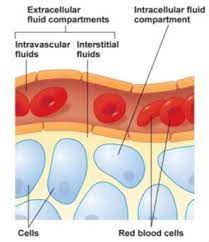
B. Extracellular Fluid (ECF)
Location: The ECF is all the fluid found outside the cells. It acts as the body's internal environment that bathes all cells.
Proportion: The ECF constitutes approximately one-third (1/3) of the TBW, which is roughly 14 liters (20% of body weight) in a 70 kg adult.
Composition - The Body's Transport Medium:
- Major Cations: Predominantly Sodium (Na⁺), which is the primary determinant of ECF osmolarity and volume.
- Major Anions: Predominantly Chloride (Cl⁻) and Bicarbonate (HCO₃⁻), a crucial component of the body's buffering system.
- Other Components: A rich soup of nutrients, gases, hormones, and waste products.
Sub-compartments of ECF:
The ECF is not a monolithic entity; it is further subdivided into several distinct yet interconnected compartments:
i. Interstitial Fluid (ISF)
This is the "tissue fluid," filling the microscopic spaces between the cells. It is the largest component of the ECF, comprising about 80% of ECF volume. Its ionic composition is similar to plasma, but it has a significantly lower protein concentration. The ISF is the critical medium for the exchange of nutrients, gases, and waste between the blood and the cells.
ii. Plasma
This is the fluid component of blood, circulating within the cardiovascular system. It accounts for about 20% of ECF volume. Its defining characteristic is its high concentration of plasma proteins (e.g., albumin). Plasma is the primary transport medium for blood cells, nutrients, hormones, and waste products.
iii. Transcellular Fluid
A small, specialized component of the ECF, representing only 1-2% of body weight. It consists of fluids secreted by specific cells into distinct, epithelial-lined spaces. The composition of these fluids is often unique and tailored to their specific function.
Examples: Cerebrospinal Fluid (CSF), Intraocular Fluid, Synovial Fluid, Serous Fluids (pleural, pericardial), and Gastrointestinal Secretions.
Fluid Movement Between Compartments and Regulatory Mechanisms
The precise movement of water and solutes between the body's fluid compartments is a cornerstone of physiological homeostasis. This dynamic equilibrium is meticulously regulated by physical forces, membrane properties, and complex neurohormonal systems.
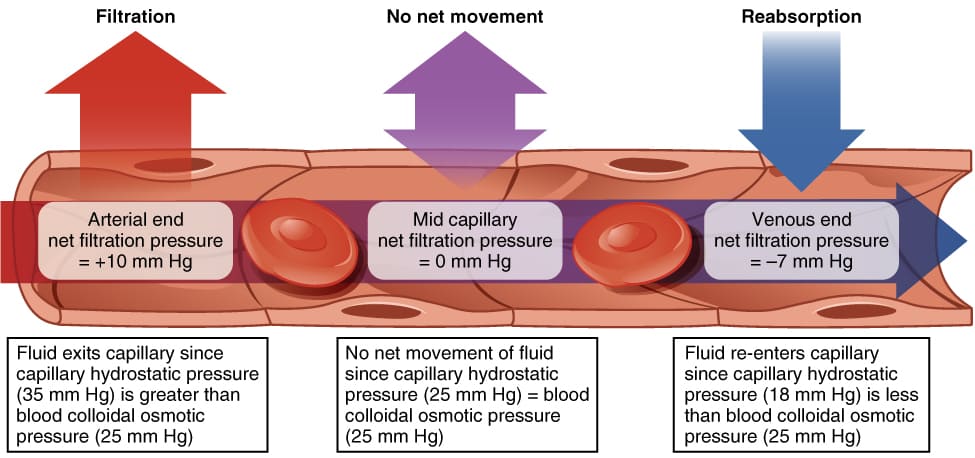
A. Fluid Movement Between Plasma and Interstitial Fluid (Across Capillary Walls)
The exchange of fluid, nutrients, gases, and waste products between the blood (plasma) and the cells (via the ISF) occurs primarily across the thin walls of the capillaries. This movement is governed by Starling Forces, which represent the interplay of hydrostatic and oncotic pressures.
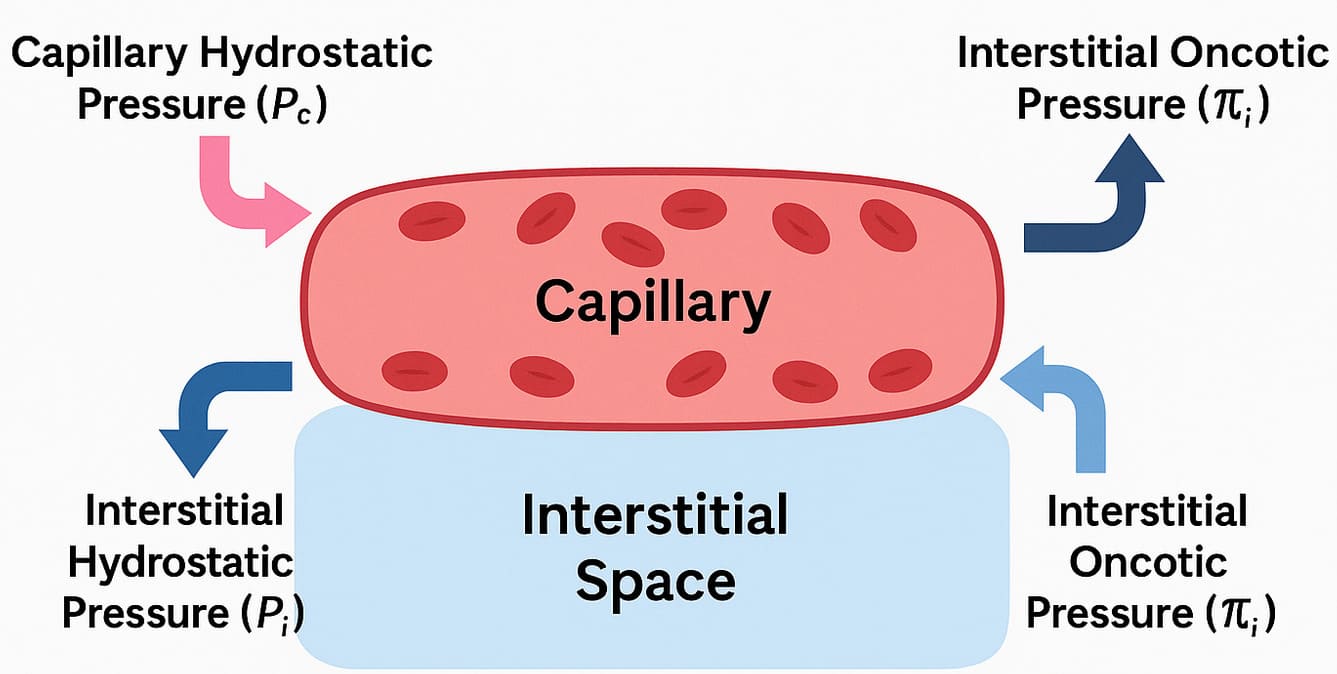 Starling Forces - The Drivers of Capillary Exchange:
Starling Forces - The Drivers of Capillary Exchange:Capillary Hydrostatic Pressure (Pc):
- Definition: This is the pressure exerted by the blood within the capillaries, effectively the "pushing" force of the blood against the capillary wall.
- Effect: It tends to force fluid out of the capillary and into the interstitial space (filtration).
- Dynamics: Pc is highest at the arterial end of the capillary (typically around 30-35 mmHg) and progressively drops to a lower value at the venous end (typically around 10-15 mmHg).
- Definition: This is the pressure exerted by the fluid in the interstitial space surrounding the capillary.
- Effect: It tends to push fluid back into the capillary.
- Dynamics: Pif is usually very low, often close to zero or even slightly negative.
- Definition: This is the osmotic pressure exerted by the large, non-diffusible proteins (primarily albumin) within the plasma.
- Effect: It tends to pull fluid into the capillary from the interstitial space (reabsorption).
- Dynamics: πc remains relatively constant along the length of the capillary (typically around 25-28 mmHg).
- Definition: This is the osmotic pressure exerted by the small amount of proteins in the interstitial fluid.
- Effect: It tends to pull fluid out of the capillary.
- Dynamics: πif is normally very low (typically 2-8 mmHg).
Net Filtration Pressure (NFP): The Sum of the Forces
The net movement of fluid is determined by the balance of these forces, expressed by the Starling equation: NFP = (Pc - Pif) - (πc - πif)
- At the arterial end: NFP = (35 - 0) - (26 - 2) = +11 mmHg. A positive NFP indicates net filtration (fluid moves out).
- At the venous end: NFP = (15 - 0) - (26 - 2) = -9 mmHg. A negative NFP indicates net reabsorption (fluid moves in).
The Lymphatic System:
There is a slight imbalance where filtration slightly exceeds reabsorption. This excess fluid and any leaked proteins are collected by the lymphatic system, which acts as a drainage system, returning this "lymph" to the circulation. This is vital for preventing interstitial edema. Failure of this system results in lymphedema.
Fluid Movement Between ECF and ICF (Across Cell Membranes)
The exchange between the ISF and the ICF is driven primarily by osmosis. The cell membrane is highly permeable to water (largely via aquaporins) but relatively impermeable to most solutes.
Osmolarity vs. Tonicity
Tonicity describes the effect a solution has on cell volume, based on its concentration of non-penetrating solutes.
- Isotonic ECF: No net movement of water; cell volume remains stable.
- Hypotonic ECF: Water moves into the cells, causing them to swell (and potentially lyse). This can cause cerebral edema.
- Hypertonic ECF: Water moves out of the cells, causing them to shrink (crenation). This can also cause severe neurological symptoms.
Active Transport's Essential Role:
While water movement is passive, the maintenance of the osmotic gradients is dependent on active transport. The Na⁺/K⁺ ATPase pump is critical. By constantly pumping 3 Na⁺ out and 2 K⁺ in, it counters the natural tendency of water to enter the cell (due to the high concentration of trapped intracellular proteins), thereby maintaining cell volume and preventing lysis.
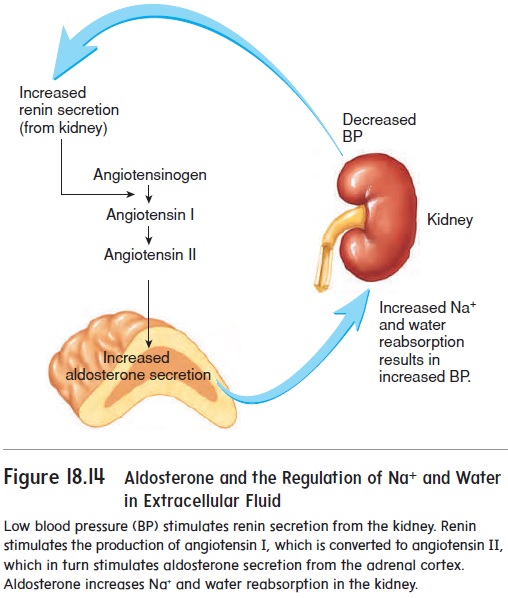
Regulation of Body Fluid Volume and Osmolarity
This is achieved through complex, interconnected neurohormonal feedback systems.
A. Regulation of ECF Volume (primarily Na⁺ balance)
ECF volume is primarily determined by its sodium content, as "where Na⁺ goes, water follows."
- Renin-Angiotensin-Aldosterone System (RAAS): Activated by low blood pressure/volume. Angiotensin II is a potent vasoconstrictor and stimulates the release of Aldosterone. Aldosterone acts on the kidneys to dramatically increase Na⁺ reabsorption, which in turn leads to water reabsorption, expanding ECF volume.
- Antidiuretic Hormone (ADH) / Vasopressin: Released in response to increased plasma osmolarity or significantly decreased blood volume. ADH increases water reabsorption in the kidneys by promoting the insertion of aquaporin channels, leading to concentrated urine.
- Atrial Natriuretic Peptide (ANP) / BNP: Released by the heart in response to high ECF volume/pressure. They are counter-regulatory, promoting Na⁺ and water excretion (natriuresis and diuresis) by the kidneys to reduce volume and pressure.
- Sympathetic Nervous System: Activation promotes Na⁺ and water retention by reducing renal blood flow and stimulating renin release.
B. Regulation of ECF Osmolarity (primarily water balance)
ECF osmolarity is primarily determined by the concentration of solutes relative to water, and is tightly controlled to stay within 280-300 mOsm/L.
- ADH (Vasopressin): The primary hormone for osmolarity regulation. Its release is exquisitely sensitive to changes in plasma osmolarity. A small increase in osmolarity strongly stimulates ADH release, leading to water retention to dilute the ECF. A decrease inhibits ADH, leading to water excretion.
- Thirst Mechanism: The behavioral component. Osmoreceptors in the hypothalamus, stimulated by increased osmolarity, create the conscious sensation of thirst, prompting water intake to dilute the ECF.
Clinical Significance of Fluid Imbalances
Disturbances in fluid regulation can have profound and life-threatening consequences.
- Hypovolemia (ECF Volume Deficit): Caused by hemorrhage, severe dehydration, or burns. Leads to decreased blood pressure, poor tissue perfusion, and can progress to hypovolemic shock.
- Hypervolemia (ECF Volume Excess): Caused by heart failure, renal failure, or cirrhosis. Leads to high blood pressure and edema. When in the lungs (pulmonary edema), it impairs gas exchange.
- Hyponatremia (Low Plasma Na⁺): A disorder of water excess. A hypotonic ECF causes water to shift into cells, leading to cellular swelling, especially in the brain (cerebral edema), which can cause seizures and coma.
- Hypernatremia (High Plasma Na⁺): A disorder of water deficit. A hypertonic ECF causes water to shift out of cells, leading to cellular shrinkage, especially in the brain, which can also cause seizures and coma.
- Edema (Excess Interstitial Fluid): Can be caused by increased capillary hydrostatic pressure (e.g., heart failure), decreased plasma oncotic pressure (e.g., liver failure), increased capillary permeability (e.g., inflammation), or impaired lymphatic drainage.
Measurement of Fluid Compartments (Indicator Dilution Method)
The volume of a compartment is calculated as: Volume = Mass of Indicator Injected / Concentration of Indicator in Sample. The key is choosing an indicator that distributes only in the target compartment.
- Total Body Water (TBW): Measured with heavy water (D₂O) or tritiated water (HTO), which distribute everywhere water does.
- Extracellular Fluid (ECF): Measured with inulin or mannitol, which cross capillaries but cannot enter cells.
- Plasma Volume: Measured with Evans blue dye or radioactive albumin, which are large molecules that cannot cross capillaries and remain in the plasma.
- Interstitial Fluid (ISF) Volume: Calculated indirectly: ISF = ECF - Plasma.
- Intracellular Fluid (ICF) Volume: Calculated indirectly: ICF = TBW - ECF.
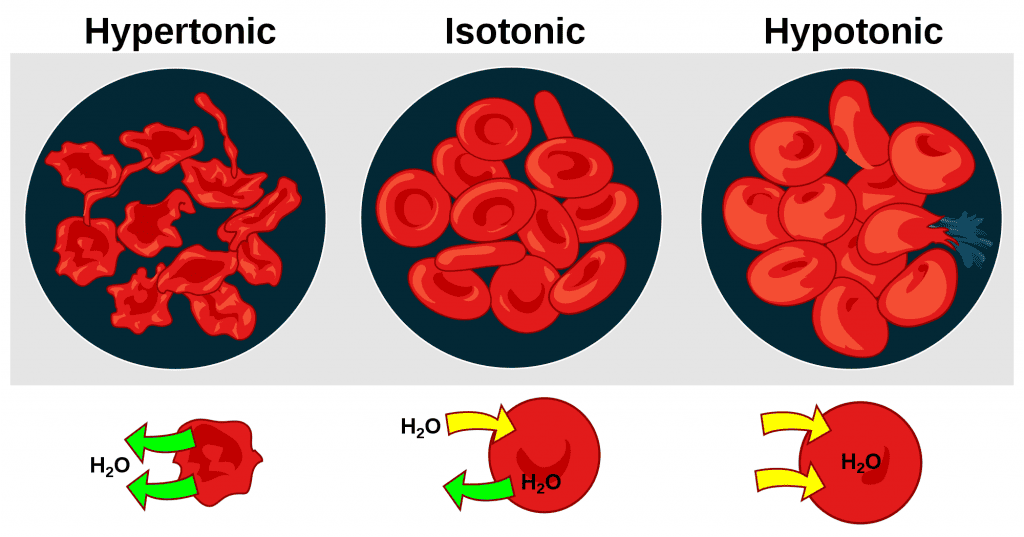
Tonicity, Osmolarity, and Clinical Implications of IV Fluids
The human body is an intricate system highly dependent on the precise balance of water and solutes across its various compartments. Understanding the concepts of osmolarity and tonicity, and their clinical implications, particularly with intravenous (IV) fluid administration, is fundamental to effective medical practice.
1. Osmolarity vs. Tonicity:
These two terms are often used interchangeably, but they possess distinct physiological meanings that are critical when considering fluid shifts across cell membranes.
Osmolarity:
- Definition: Osmolarity quantifies the total concentration of all solute particles present in a solution, expressed as milliosmoles per liter of solution (mOsm/L).
- "Effective" vs. "Ineffective" Osmoles:
- Effective Osmoles (Non-penetrating Solutes): Solutes that cannot readily cross a cell membrane and thus exert an osmotic force. Examples include Na⁺, Cl⁻, HCO₃⁻, and mannitol.
- Ineffective Osmoles (Penetrating Solutes): Solutes that can readily cross the cell membrane and therefore do not contribute to sustained osmotic gradients. Examples include urea and ethanol.
- Physiological Reference: Normal plasma osmolarity is tightly regulated between 280-300 mOsm/L.
Tonicity:
- Definition: Tonicity is a functional term describing the effect a solution has on cell volume, determined solely by the concentration of non-penetrating solutes.
- Types of Tonicity:
- Isotonic: A solution with the same concentration of non-penetrating solutes as the cell's cytoplasm. No net water movement occurs, and cell volume remains stable. (e.g., 0.9% Normal Saline, Lactated Ringer's).
- Hypotonic: A solution with a lower concentration of non-penetrating solutes. Water moves into cells, causing them to swell and potentially lyse. (e.g., 0.45% Saline, D5W after glucose metabolism).
- Hypertonic: A solution with a higher concentration of non-penetrating solutes. Water moves out of cells, causing them to shrink (crenation). (e.g., 3% Saline, D5NS, Mannitol).
Key Difference (Why it matters):
A solution can be isosmotic but hypotonic. A classic example is 5% Dextrose in Water (D5W). Initially, its osmolarity is ~252 mOsm/L (isosmotic). However, once cells metabolize the glucose, it leaves behind pure water, which is hypotonic to cells, causing water to shift into them. Therefore, tonicity, not just osmolarity, is what truly matters for predicting cell volume changes.
2. Importance of Maintaining Fluid Osmolarity and Tonicity
- Cellular Function: All cells depend on a stable intracellular volume and extracellular environment.
- Enzyme Activity: Enzymes are highly sensitive to changes in cell volume, pH, and ion concentrations.
- Membrane Potential: The electrochemical gradients crucial for nerve and muscle function rely on stable environments.
- Brain Function: Neurons are exquisitely vulnerable to osmotic shifts. Swelling (cerebral edema in hyponatremia) or shrinking (in hypernatremia) can lead to severe neurological dysfunction, seizures, and death.
- Circulatory Function: ECF volume, particularly plasma volume, directly impacts blood pressure and tissue perfusion.
3. Effects of External Factors on Fluid Compartments: IV Fluids
Their safe and effective administration requires a deep understanding of their tonicity and how they distribute.
General Principles of IV Fluid Distribution:
- Initial Introduction: All IV fluids are introduced directly into the plasma.
- Subsequent Distribution: Depends entirely on the fluid's tonicity.
- Therapeutic Goal: Isotonic fluids expand ECF volume; hypotonic fluids shift water into cells; hypertonic fluids draw water out of cells.
A. Isotonic Solutions (e.g., 0.9% Normal Saline, Lactated Ringer's)
- Distribution: They do not cause a significant net shift of water into or out of cells. Therefore, they primarily expand the Extracellular Fluid (ECF) compartment. For every 1L infused, ~250-300 mL remains in the plasma and ~700-750 mL moves into the interstitial fluid.
- Clinical Uses: Volume resuscitation in hypovolemic shock, severe dehydration, and burns.
- Hospital Scenario: A hypotensive trauma patient with acute blood loss is given a rapid IV infusion of 1-2 liters of Normal Saline or Lactated Ringer's to rapidly increase circulating blood volume and raise blood pressure.
B. Hypotonic Solutions (e.g., 0.45% Saline, D5W after glucose metabolism)
- Distribution: Hypotonic solutions cause water to shift from the ECF into the Intracellular Fluid (ICF).
- Clinical Uses: Treating hypernatremia (cellular dehydration) and providing free water replacement.
- Hospital Scenario: A patient with severe hypernatremia has their brain cells rehydrated via a slow and controlled infusion of 0.45% Saline or D5W. This must be done slowly to avoid causing cerebral edema.
C. Hypertonic Solutions (e.g., 3% Saline, Mannitol)
- Distribution: These create a powerful osmotic gradient that draws water out of the ICF and into the ECF, expanding the ECF at the expense of the ICF.
- Clinical Uses: Treating severe, symptomatic hyponatremia (to pull water out of swollen brain cells) and reducing cerebral edema from conditions like traumatic brain injury.
- Hospital Scenario: A patient with severe hyponatremia and seizures is given small, controlled boluses of 3% Saline to rapidly reduce brain swelling. Extreme caution is required to avoid Osmotic Demyelination Syndrome (ODS) from too-rapid correction.
4. Effects of Blood Transfusion
Products like packed red blood cells (PRBCs) are considered isotonic. Their distribution primarily expands the intravascular compartment (plasma volume) and directly increases the oxygen-carrying capacity of the blood.
5. Colloids vs. Crystalloids
Crystalloids:
- Definition: Aqueous solutions of small, water-soluble molecules (e.g., Normal Saline, Lactated Ringer's).
- Distribution: Can freely cross capillary membranes and distribute throughout the entire ECF.
- Advantages: Inexpensive and effective for general ECF volume expansion.
- Disadvantages: A large volume is needed for sustained plasma expansion as much of it moves into the interstitial space, which can cause significant edema.
Colloids:
- Definition: Solutions containing large molecules (e.g., albumin, starches) that do not readily cross intact capillary membranes.
- Distribution: Primarily remain within the intravascular compartment (plasma), exerting oncotic pressure that helps retain or pull fluid into the blood vessels.
- Advantages: More effective at expanding plasma volume per unit infused.
- Disadvantages: More expensive, potential for allergic reactions, and concerns about kidney injury with some synthetic colloids.
Summary of Fluid Shifts and Clinical Implications
| IV Fluid Type | Tonicity | Final Distribution | Effect on Cells | Primary Clinical Use |
|---|---|---|---|---|
| Isotonic | Isotonic | Expands ECF (Plasma + ISF) | No change | ECF volume expansion (shock, dehydration) |
| Hypotonic | Hypotonic | Shifts from ECF to ICF | Swell | Cellular rehydration (hypernatremia) |
| Hypertonic | Hypertonic | Shifts from ICF to ECF | Shrink | Reduce cerebral edema, treat severe hyponatremia |
| Colloids | Isotonic | Primarily remains in Plasma | No change | Plasma volume expansion (severe shock) |
| Blood Products | Isotonic | Primarily remains in Plasma | No change | Replace blood loss, improve O₂ carrying capacity |
Solutes, Solvents, and Simple Movement in Body Fluids
At the heart of all physiological processes involving fluids is the interaction between solutes and solvents, and their movement across various compartments.
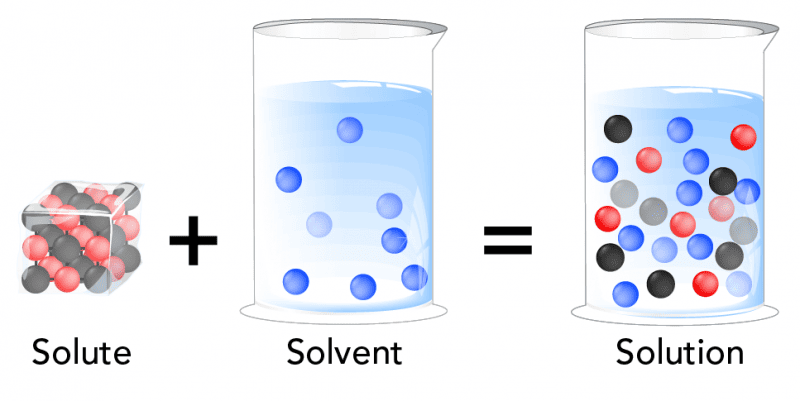
1. Solutes and Solvents: The Basics
- Solution: A homogeneous mixture composed of two or more substances.
- Solvent: The substance that is present in the greatest amount in a solution and does the dissolving.
- Solute: The substance(s) that are present in a lesser amount in a solution and get dissolved by the solvent.
What is the Solvent of Body Fluid?
The primary and overwhelmingly abundant solvent in all body fluids is WATER (H₂O).
Water's unique properties make it an ideal biological solvent:
- Polarity: Allows it to dissolve a wide variety of other polar molecules and ions.
- High Heat Capacity: Helps regulate body temperature.
- High Heat of Vaporization: Allows for cooling through sweating.
Common Solutes in Body Fluids:
Body fluids are complex solutions containing a vast array of solutes:
- Electrolytes: Ions that conduct electricity.
- Cations (positively charged): Sodium (Na⁺), Potassium (K⁺), Calcium (Ca²⁺), Magnesium (Mg²⁺).
- Anions (negatively charged): Chloride (Cl⁻), Bicarbonate (HCO₃⁻), Phosphate (HPO₄²⁻).
- Non-electrolytes:
- Nutrients: Glucose, amino acids, fatty acids, vitamins.
- Metabolic Wastes: Urea, creatinine, uric acid.
- Proteins: Albumin, globulins, fibrinogen.
- Gases: Oxygen (O₂), Carbon Dioxide (CO₂).
2. Simple Movement of Solutes and Solvents
The movement of substances is primarily governed by passive processes that do not require cellular energy (ATP).
A. Movement of Solutes: Diffusion
- Definition: The net movement of solute particles from an area of higher solute concentration to an area of lower solute concentration (down the concentration gradient).
- Mechanism: Driven by the inherent random kinetic energy of molecules.
- Factors Affecting Diffusion Rate: The rate is faster with a larger concentration gradient, higher temperature, smaller molecular size, shorter distance, and larger surface area.
- Types of Diffusion:
- Simple Diffusion: Solutes pass directly through the lipid bilayer (e.g., O₂, CO₂, fatty acids).
- Facilitated Diffusion: Solutes move with the help of membrane proteins (channels or carriers), still following the concentration gradient (e.g., glucose, ions).
B. Movement of Solvents: Osmosis
- Definition: The net movement of water (the solvent) across a selectively permeable membrane from an area of higher water concentration (lower solute) to an area of lower water concentration (higher solute).
- Mechanism: Water molecules move down their own concentration gradient.
- Selectively Permeable Membrane: Crucial for osmosis, as it allows water to pass but restricts most solutes.
- Osmotic Pressure: The pressure needed to prevent the inward flow of water across a semipermeable membrane. The higher the solute concentration, the higher the osmotic pressure.
Summary of Movement Principles:
- Solutes move by Diffusion: From high solute concentration to low solute concentration.
- Water (Solvent) moves by Osmosis: From high water concentration (low solute) to low water concentration (high solute).
These passive movements are essential for:
- Nutrient delivery and waste removal.
- Gas exchange in the lungs.
- Maintaining cell volume and shape.
- Fluid balance between intracellular and extracellular compartments.
Clinical Scenarios:
Basic Principle: Water follows solutes. Specifically, water moves from an area of lower effective solute concentration (higher water concentration) to an area of higher effective solute concentration (lower water concentration) across a semipermeable membrane.
Scenario 1: Blood Transfusion
- Product: Whole blood or packed red blood cells.
- Tonicity: Isotonic.
- Effect: Primarily increases the plasma volume. No significant shift of fluid between ECF and ICF. Also delivers oxygen-carrying capacity.
- Clinical Use: To replace blood loss or treat anemia.
Scenario 2: Intravenous (IV) Fluid Administration
1. Isotonic Solutions (e.g., Normal Saline - 0.9% NaCl, Lactated Ringer's - LR)
- Composition: 0.9% NaCl (NS) contains 154 mEq/L Na⁺ and 154 mEq/L Cl⁻. Lactated Ringer's (LR) contains Na⁺, Cl⁻, K⁺, Ca²⁺, and lactate. Both are effectively isotonic.
- Distribution: The fluid stays entirely within the ECF compartment, distributing between the plasma (~1/4) and interstitial fluid (~3/4).
- Clinical Uses: Volume expansion for dehydration, hypovolemic shock, hemorrhage.
- Hospital Scenario: A hypotensive car accident patient receives a rapid infusion of NS or LR to restore intravascular volume and blood pressure.
2. Hypotonic Solutions (e.g., 0.45% NaCl - Half Normal Saline, D5W - Dextrose 5% in Water)
- Composition: 0.45% NaCl has half the sodium of NS. D5W is initially isotonic, but the dextrose is rapidly metabolized, leaving free water.
- Distribution: Water moves from the ECF into the ICF compartment to equalize osmolality, hydrating the cells.
- Clinical Uses: To treat cellular dehydration (e.g., hypernatremia).
- Hospital Scenario: A patient with severe hypernatremia is given a slow infusion of Half Normal Saline to allow water to shift into their dehydrated brain cells.
3. Hypertonic Solutions (e.g., 3% NaCl - Hypertonic Saline, D5NS)
- Composition: 3% NaCl is very hypertonic (1026 mOsm/L). D5NS is initially hypertonic, then becomes isotonic as dextrose is metabolized.
- Distribution: Water moves out of the ICF and into the ECF compartment, causing cells to shrink.
- Clinical Uses: To treat severe symptomatic hyponatremia and to reduce cerebral edema.
- Hospital Scenario: A patient with traumatic brain injury and high intracranial pressure is given a slow infusion of 3% Hypertonic Saline to draw fluid out of the swollen brain cells.
4. Colloids (e.g., Albumin, Dextran, Hetastarch)
- Composition: Solutions containing large molecules (proteins, large sugars) that do not easily cross capillary membranes.
- Distribution: Due to their large size, they primarily remain within the intravascular space (plasma), exerting an oncotic pull that draws fluid from the interstitial space into the plasma.
- Clinical Uses: Rapid plasma volume expansion, especially in severe hypoalbuminemia or burns.
- Hospital Scenario: A patient with severe burns and plasma volume depletion is given an infusion of Albumin to rapidly restore intravascular volume.
Summary Table of IV Fluid Effects:
| IV Fluid Type | Effective Tonicity | Primary Distribution | Effect on ICF Cells |
|---|---|---|---|
| Isotonic (NS, LR) | Isotonic | ECF only (plasma & ISF) | No change |
| Hypotonic (0.45% NaCl, D5W) | Hypotonic | ECF & ICF | Swell |
| Hypertonic (3% NaCl) | Hypertonic | ECF (draws from ICF) | Shrink |
| Colloids (Albumin) | Effectively Hypertonic (oncotic) | Plasma only (draws from ISF) | No direct effect |
Test Your Knowledge
A quiz on Body Fluids, Osmolarity, Tonicity & IV Solutions.
1. Which of the following best defines osmolarity?
- The concentration of non-penetrating solutes in a solution.
- The effect a solution has on cell volume.
- The total concentration of all solute particles in a solution.
- The pressure required to stop water movement across a membrane.
Correct (c): Osmolarity measures the sum of all solute particles, both penetrating (ineffective) and non-penetrating (effective), in a given volume of solution.
Incorrect (a, b): This defines tonicity.
Incorrect (d): This describes osmotic pressure.
2. A solution with a lower concentration of non-penetrating solutes than the cell's cytoplasm is described as:
- Isosmotic
- Isotonic
- Hypotonic
- Hypertonic
Correct (c): Hypotonic solutions have fewer non-penetrating solutes, causing water to move into cells and make them swell.
Incorrect (b): Isotonic solutions have the same concentration, causing no change in cell volume.
Incorrect (d): Hypertonic solutions have a higher concentration, causing cells to shrink.
3. Which solute is generally considered an ineffective osmole in the context of sustained osmotic gradients across cell membranes?
- Sodium (Na+)
- Glucose
- Urea
- Mannitol
Correct (c): Urea readily crosses most cell membranes, so it does not create a sustained osmotic gradient and is an ineffective osmole.
Incorrect (a): Sodium is the primary effective osmole in the ECF.
Incorrect (d): Mannitol is specifically designed not to cross membranes, making it a potent effective osmole.
4. Normal plasma osmolarity is approximately:
- 150-200 mOsm/L
- 280-300 mOsm/L
- 350-400 mOsm/L
- 450-500 mOsm/L
Correct (b): This is the tightly regulated normal range for plasma osmolarity in humans.
Incorrect: The other ranges are either too low or too high for a healthy state.
5. When a cell is placed in a hypertonic solution, what will happen to the cell?
- It will swell and potentially lyse.
- It will remain stable in volume.
- It will shrink (crenation).
- It will undergo active transport of water.
Correct (c): In a hypertonic solution, the ECF has more non-penetrating solutes, pulling water out of the cell via osmosis and causing it to shrink.
Incorrect (a): This happens in a hypotonic solution.
Incorrect (b): This happens in an isotonic solution.
Incorrect (d): Water moves passively by osmosis.
6. A patient with severe hypovolemic shock requires rapid fluid resuscitation. Which IV fluid is most appropriate?
- 0.45% Saline
- D5W
- 3% Saline
- Lactated Ringer's
Correct (d): Isotonic crystalloids like Lactated Ringer's are first-line for hypovolemic shock because they expand the extracellular fluid volume without causing dangerous fluid shifts.
Incorrect (a, b): These are hypotonic and would shift water into cells, worsening intravascular depletion.
Incorrect (c): This is hypertonic and used for specific conditions like cerebral edema, not routine resuscitation.
7. How does 5% Dextrose in Water (D5W) behave clinically after the glucose is metabolized?
- It becomes hypertonic.
- It primarily expands the intravascular compartment.
- It acts as a hypotonic solution, providing free water.
- It acts as an isotonic solution long-term.
Correct (c): Once the glucose is metabolized, it leaves behind pure water. This "free water" then moves into cells due to osmosis, effectively acting as a hypotonic solution and rehydrating cells.
8. What is a primary clinical indication for administering a hypertonic saline solution (e.g., 3% NaCl)?
- Correcting hypernatremia.
- Treating severe symptomatic hyponatremia with cerebral edema.
- Routine maintenance fluid.
- Expanding interstitial fluid volume.
Correct (b): Hypertonic saline is used to rapidly raise ECF sodium and pull water out of swollen brain cells in life-threatening hyponatremia.
Incorrect (a): Hypernatremia is treated with hypotonic solutions.
Incorrect (c): It is a high-risk fluid, not for routine use.
9. What is the main advantage of colloids over crystalloids for plasma volume expansion?
- Colloids are less expensive.
- Colloids distribute throughout the entire ECF.
- Colloids are more effective at expanding plasma volume per unit infused.
- Colloids are primarily used for cellular rehydration.
Correct (c): Due to their large molecules remaining in the intravascular space and exerting oncotic pressure, colloids expand plasma volume with a smaller amount of fluid compared to crystalloids.
Incorrect (a): Colloids are significantly more expensive.
Incorrect (b): Crystalloids distribute throughout the ECF; colloids largely stay in the plasma.
10. The primary solvent in all human body fluids is:
- Sodium chloride
- Plasma proteins
- Water
- Glucose
Correct (c): Water is the universal solvent for biological systems, making up the vast majority of all body fluids.
Incorrect: The other options are important solutes, not the solvent.
11. The net movement of solute particles from an area of higher to lower concentration is called:
- Osmosis
- Active Transport
- Diffusion
- Filtration
Correct (c): Diffusion is the passive movement of solute particles down their concentration gradient.
Incorrect (a): Osmosis is the movement of water (the solvent).
Incorrect (b): Active transport requires energy to move solutes against a gradient.
12. Which type of diffusion requires membrane proteins but not ATP?
- Simple Diffusion
- Facilitated Diffusion
- Active Transport
- Endocytosis
Correct (b): Facilitated diffusion uses membrane proteins (channels or carriers) to help solutes move down their gradient, without ATP.
Incorrect (a): Simple diffusion does not require proteins.
Incorrect (c): Active transport requires ATP.
13. A patient with severe hypernatremia would most likely benefit from which type of IV fluid?
- Isotonic crystalloid
- Hypertonic saline
- Hypotonic solution
- Colloid
Correct (c): In hypernatremia, the ECF is hypertonic, causing cells to shrink. A hypotonic solution will dilute the ECF sodium and cause water to move back into the cells, rehydrating them.
14. What is the approximate distribution of 1 liter of an isotonic crystalloid (like Normal Saline) after infusion?
- All 1L remains in the intravascular space.
- All 1L shifts into the intracellular fluid.
- ~250 mL intravascular, ~750 mL interstitial.
- ~500 mL intravascular, ~500 mL intracellular.
Correct (c): Isotonic crystalloids distribute throughout the entire ECF. Since the ECF is roughly 1/4 plasma and 3/4 interstitial fluid, an infused liter will partition accordingly.
15. Why are brain cells particularly vulnerable to rapid shifts in ECF osmolarity?
- They produce less ATP than other cells.
- They are in a rigid skull with limited room for expansion.
- Their cell membranes are impermeable to water.
- They only contain ineffective osmoles.
Correct (b): The brain's enclosure within the skull means that significant swelling (from hypotonicity) or shrinking (from hypertonicity) can lead to severe neurological damage.
16. The term describing the effect a solution has on cell volume is _________.
17. In osmosis, water moves toward an area of _________ solute concentration.
18. _________ are solutions with large molecules that primarily remain within the intravascular compartment.
19. The primary cation in the ECF that is a major effective osmole is _________.
20. When a cell is placed in a hypotonic solution, it will _________.
Quiz Complete!
Your Score:
0%
0 / 0 correct