Acids, Bases, pH and Buffer : The Chemical Environment
Objectives
At the end of this section, you will be able to understand:
- Acids, Bases, and pH:
- What are acids and bases?
- What is pH? The pH scale.
- Buffers: Why they are crucial in living systems.
- Why it's important: Biochemical reactions are very sensitive to pH. Maintaining the correct pH is vital for survival.
Acids and Bases
The environment within and around our cells is not static; it's a dynamic chemical soup where countless reactions occur simultaneously. Just like a baker needs to precisely control oven temperature, the "chemical temperature" of our biological systems – its acidity or basicity – must be meticulously maintained within an incredibly narrow range. This control, measured by pH, is paramount for life. Even minor deviations can lead to catastrophic consequences, as the delicate structures of proteins and enzymes are exquisitely sensitive to pH changes. This maintenance of a stable internal pH is a cornerstone of homeostasis.
What Makes Something Acidic or Basic? It's All About the Proton (H⁺)
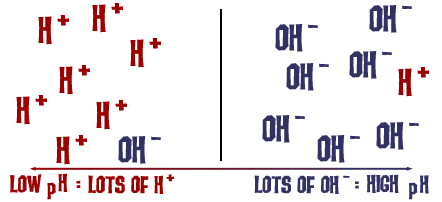
At the heart of acidity and basicity is one tiny, yet profoundly powerful, particle: the hydrogen ion (H⁺). A hydrogen ion (H⁺) is essentially just a proton. The concentration of these free H⁺ ions in a solution is the ultimate determinant of whether that solution is acidic, neutral, or basic.
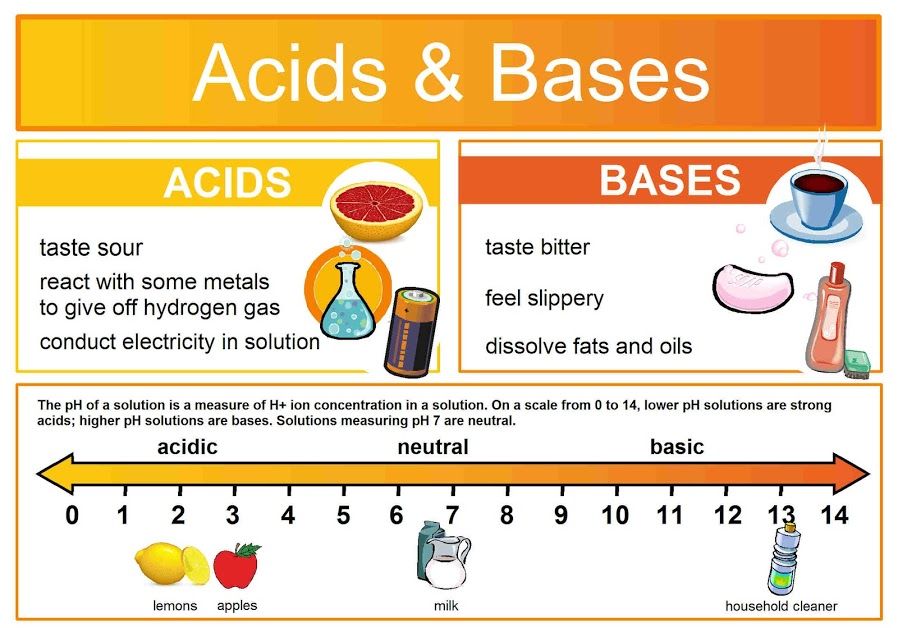
Acids: The Proton Donors
An acid is defined as any substance that, when dissolved in an aqueous solution, releases or donates hydrogen ions (H⁺), thereby increasing the concentration of free H⁺ in that solution.
Strength: A strong acid dissociates almost completely in water, releasing nearly all its H⁺ ions. A weak acid only partially dissociates.
Real-World and Physiological Examples:
- Hydrochloric Acid (HCl): A strong acid in your stomach, crucial for digestion. It undergoes almost complete dissociation:
HCl(aq) → H⁺(aq) + Cl⁻(aq) - Carbonic Acid (H₂CO₃): A crucial weak acid in your blood. It only partially dissociates, maintaining an equilibrium:
H₂CO₃(aq) ⇌ H⁺(aq) + HCO₃⁻(aq)
The double arrow (⇌) indicates the reaction is reversible.
Bases: The Proton Acceptors
A base (or alkali) is any substance that, when dissolved in an aqueous solution, decreases the concentration of H⁺ ions by "accepting" them or by releasing hydroxide ions (OH⁻).
Strength: A strong base dissociates almost completely. A weak base only partially accepts H⁺ or releases OH⁻ ions.
Real-World and Physiological Examples:
- Sodium Hydroxide (NaOH): A very strong base. It dissociates completely:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
The released OH⁻ then rapidly combines with H⁺ to form water:OH⁻(aq) + H⁺(aq) → H₂O(l) - Bicarbonate (HCO₃⁻): The most important weak base in blood plasma. It can readily accept a free H⁺ ion to form carbonic acid, "soaking up" excess acid:
HCO₃⁻(aq) + H⁺(aq) ⇌ H₂CO₃(aq)
The Importance of "Aqueous Solution"
The definition of acids and bases in this context relies on their behavior in aqueous solutions (where water is the solvent). Water itself can slightly dissociate: H₂O(l) ⇌ H⁺(aq) + OH⁻(aq). In pure water, the concentrations of H⁺ and OH⁻ are equal, making it neutral. Acids disturb this balance by increasing H⁺, and bases disturb it by decreasing H⁺.
Clinical Significance for Nurses: Why Acid-Base Balance is Critical
Understanding acids and bases is not just theoretical; it's fundamental to clinical practice:
- pH Homeostasis: The body meticulously maintains the pH of arterial blood between 7.35 and 7.45. Even slight deviations (e.g., acidosis <7.35 or alkalosis >7.45) can impair enzyme function, alter protein structures, disrupt electrolyte balance (e.g., potassium levels), and depress or overstimulate the central nervous system, potentially leading to organ failure and death.
- Buffer Systems: The body employs sophisticated buffer systems (like the bicarbonate buffer system, phosphate buffer system, and protein buffer system) to resist sudden changes in pH. These buffers are mixtures of weak acids and their conjugate bases (or weak bases and their conjugate acids) that can absorb excess H⁺ or release H⁺ as needed.
- Respiratory and Renal Regulation: The lungs regulate pH by controlling the exhalation of carbon dioxide (which forms carbonic acid in blood), while the kidneys regulate pH by reabsorbing bicarbonate and excreting H⁺ ions.
- Disease States: Many disease states, such as diabetic ketoacidosis, chronic obstructive pulmonary disease (COPD), renal failure, and sepsis, are characterized by severe acid-base imbalances that nurses must be able to recognize, monitor, and assist in managing.
- Medication Administration: The pH of intravenous fluids and medications must often be carefully considered to prevent local irritation or systemic acid-base disturbances.
The pH Scale:
A Precise and Powerful Ruler for Acidity
While discussing "hydrogen ion concentration" ([H⁺]) is chemically precise, it's cumbersome. To simplify this, scientists developed the pH scale – a brilliant shorthand that transforms these unwieldy numbers into an easy-to-use linear scale.
What Does pH Stand For?
pH literally stands for "potential of Hydrogen" or "power of Hydrogen." It is a numerical scale that quantifies the concentration of hydrogen ions (H⁺) in a solution.
The Mathematical Definition
The pH is defined as the negative base-10 logarithm of the hydrogen ion concentration (in moles per liter, M):
pH = −log₁₀[H⁺]
The log₁₀ function makes large numbers manageable, and the negative sign (−) converts the negative results into the positive numbers we see on the scale.
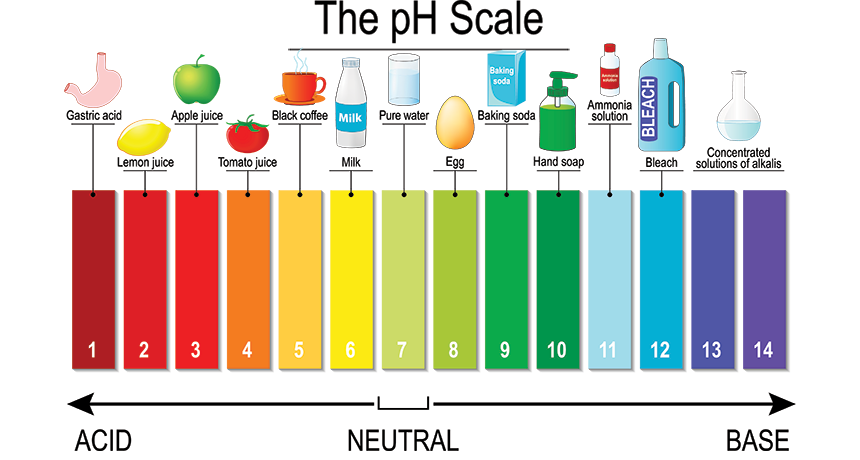
The pH Scale Range and Interpretations:
The pH scale typically ranges from 0 to 14.
Acidic (pH < 7)
The lower the pH, the higher the [H⁺] concentration.
Examples: Stomach acid (pH 1.5-3.5), lemon juice, coffee.
Neutral (pH = 7)
The concentration of H⁺ equals the concentration of OH⁻.
Examples: Pure water, human tears.
Basic/Alkaline (pH > 7)
The higher the pH, the lower the [H⁺] concentration.
Examples: Baking soda, ammonia, bleach.
The Logarithmic Nature: A Crucial Detail for Nurses
This is perhaps the most important concept about the pH scale. It is logarithmic, not linear. This means that a change of 1 pH unit represents a 10-fold (ten times) change in the actual concentration of H⁺ ions.
Applying the Principle:
- A solution with a pH of 5 is 10 times more acidic than a solution with a pH of 6.
- A solution with a pH of 4 is 100 times more acidic (10 x 10) than a solution with a pH of 6.
- A solution with a pH of 3 is 1,000 times more acidic (10 x 10 x 10) than a solution with a pH of 6.
Biological and Clinical Significance: Small pH Changes, BIG Impact
Because of this logarithmic nature, even a seemingly small numerical change in pH represents an enormous alteration in the actual concentration of H⁺ ions. This has profound implications for human physiology:
- Enzyme Function: Proteins and enzymes are highly sensitive to pH. Even a change of 0.1-0.2 pH units can significantly decrease enzyme activity. Extreme changes cause irreversible denaturation.
- Blood pH - A Tightrope Walk: The pH of human arterial blood is tightly regulated between 7.35 and 7.45. A drop from 7.4 to 7.1 means the blood is twice as acidic and is a critical medical emergency (acidosis).
- Electrolyte Balance: Changes in pH can affect how proteins bind to ions like calcium (Ca²⁺) and potassium (K⁺). For instance, acidosis can cause hyperkalemia (high potassium levels).
- Oxygen Transport: The affinity of hemoglobin for oxygen is affected by pH (the Bohr effect). Acidosis can impair overall oxygen uptake in the lungs.
- Nervous System Function: Both severe acidosis and alkalosis can profoundly affect the central nervous system, leading to symptoms ranging from confusion and lethargy to seizures and coma.
Buffers: The Body's pH "Shock Absorbers"
Our bodies are biochemical factories, constantly generating acidic or basic byproducts. If these were allowed to accumulate unchecked, the pH of our internal fluids would plummet, and life-sustaining reactions would halt. This catastrophic scenario is prevented by ingenious chemical systems known as buffers.
What is a Buffer? The Analogy
A buffer is a chemical system designed to resist significant changes in pH when an acid or a base is added. Think of buffers as the suspension system in a car. When you hit a pothole (an influx of acid), the suspension absorbs the impact, keeping the ride smooth and stable (the pH stable). Without buffers, every metabolic acid load would send the body into a pH crisis.
The Chemical Nature of a Buffer System
A buffer system is composed of a pair of molecules: a weak acid and its corresponding conjugate weak base. This pairing allows it to neutralize both excess acid and excess base.
- When an Acid (H⁺) is Added: The weak base component binds to the incoming excess H⁺ ions, taking them out of solution and preventing a sharp drop in pH.
- When a Base (OH⁻) is Added: The weak acid component releases its own H⁺ ions into the solution to replace those consumed by the base, preventing the pH from rising.
The Most Important Buffer System in Human Blood: The Carbonic Acid-Bicarbonate System
This is the body's most crucial extracellular buffer. It relies on the interplay between:
- Weak Acid: Carbonic acid (H₂CO₃)
- Conjugate Weak Base: Bicarbonate ion (HCO₃⁻)
The system works through reversible reactions:
CO₂(g) + H₂O(l) ⇌ H₂CO₃(aq)H₂CO₃(aq) ⇌ H⁺(aq) + HCO₃⁻(aq)
How it Counteracts pH Changes:
- If blood pH drops (acidosis): The bicarbonate ions (HCO₃⁻) act as the weak base and bind to the excess H⁺ ions, removing them from the solution.
- If blood pH rises (alkalosis): The carbonic acid (H₂CO₃) acts as the weak acid and dissociates, releasing H⁺ ions back into the solution.
Buffer Capacity: Limitations of the System
It's vital to understand that buffers have a limited capacity. Buffer capacity refers to the amount of acid or base a buffer can neutralize before its pH changes significantly. Once the buffer components are used up, the buffer "breaks," and pH will shift dramatically. This is why severe conditions like Diabetic Ketoacidosis (DKA) are life-threatening—the body produces so much acid that the buffer systems become exhausted.
Other Important Buffer Systems in the Body:
- Phosphate Buffer System: Important intracellularly and in urine.
- Protein Buffer System: The most abundant buffers in the body. Amino acids have groups that can both release and accept H⁺. Hemoglobin is an excellent example.
Why Health Workers MUST Understand This: Clinical Imperatives
The control of pH is a direct matter of life and death. The strict maintenance of blood pH between 7.35 and 7.45 is non-negotiable for survival.
Diagnosing and Managing Acidosis & Alkalosis:
- Acidosis (pH < 7.35): Occurs from too much acid or loss of base. Causes include Respiratory Acidosis (e.g., from COPD, hypoventilation) and Metabolic Acidosis (e.g., from DKA, lactic acid, kidney failure).
- Alkalosis (pH > 7.45): Occurs from too much base or loss of acid. Causes include Respiratory Alkalosis (e.g., from hyperventilation) and Metabolic Alkalosis (e.g., from severe vomiting).
Interpreting Arterial Blood Gas (ABG) Tests:
Nurses frequently interpret ABG results, which measure blood pH, PCO₂ (respiratory component), and HCO₃⁻ (metabolic component). Understanding buffers is essential to analyze these values, identify the primary disturbance, and evaluate the body's compensatory mechanisms.
Understanding Disease Pathophysiology:
- Diabetic Ketoacidosis (DKA): The body produces acidic "ketone bodies" at an overwhelming rate, consuming the bicarbonate buffer and leading to severe metabolic acidosis.
- Kidney Failure: Impaired kidneys cannot excrete acids or regenerate bicarbonate, leading to progressive metabolic acidosis.
- COPD: Impaired ventilation leads to chronic CO₂ retention and respiratory acidosis.
Protecting Enzymes and Proteins:
Buffers ensure that the optimal pH range for every enzyme and protein is maintained, allowing these crucial biological catalysts and structural components to perform their functions correctly.
4. Specific Biological Buffer Systems
Now that we understand the critical importance of maintaining a stable pH, let's delve into the specific buffer systems that allow the human body to achieve this remarkable feat. These systems are strategically located and exquisitely designed to work in concert, forming a robust defense network.
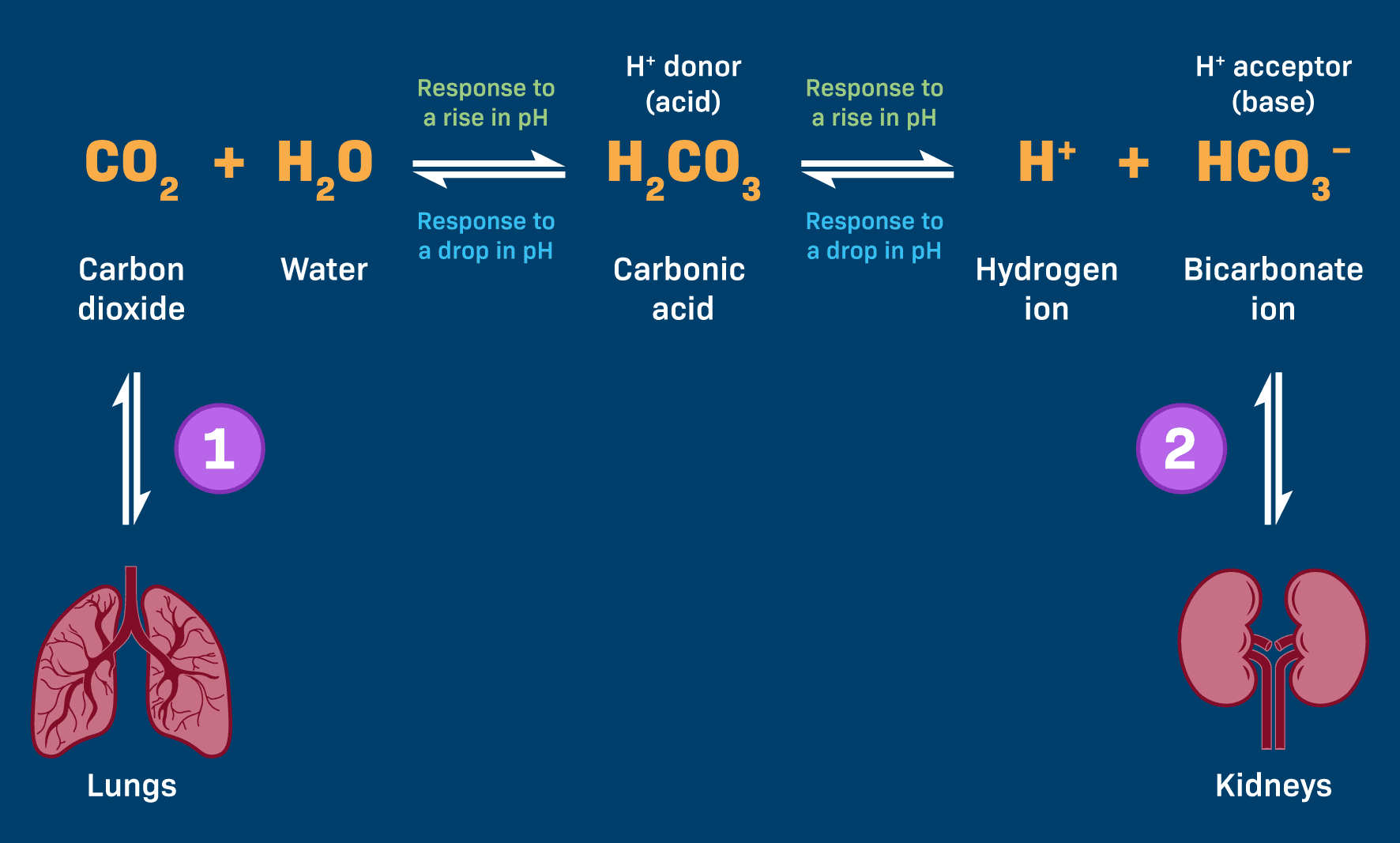
1. The Bicarbonate Buffer System: The Predominant Regulator of Extracellular Fluid pH
This is arguably the most significant buffer system in the extracellular fluid (ECF). Its power stems from its abundance, the ease with which its components can be regulated, and its intimate connections to both the respiratory (lungs) and renal (kidneys) systems.
The Components:
- Weak Acid: Carbonic Acid (H₂CO₃)
- Conjugate Weak Base: Bicarbonate Ion (HCO₃⁻)
The Dynamic Equilibrium – The Chemical Core:
These two components exist in a dynamic, reversible equilibrium:
CO₂(g) + H₂O(l) ⇌ H₂CO₃(aq) ⇌ H⁺(aq) + HCO₃⁻(aq)
Step 1: The Formation of Carbonic Acid (H₂CO₃) – Linking Metabolism to pH
Every cell produces CO₂ as a waste product. This CO₂ dissolves in blood plasma and, with the help of the enzyme carbonic anhydrase (CA), rapidly reacts with water to form carbonic acid.
CO₂ + H₂O ⇌ H₂CO₃
This step highlights that our body's ongoing metabolic activity directly contributes to the level of the weak acid in this vital buffer system.
Step 2: Dissociation of Carbonic Acid (H₂CO₃) – The Proton Donor/Acceptor Mechanism
Carbonic acid is a weak acid and maintains an equilibrium with its dissociated components: a hydrogen ion (H⁺) and a bicarbonate ion (HCO₃⁻), which is the conjugate weak base ready to accept H⁺ ions.
H₂CO₃ ⇌ H⁺ + HCO₃⁻
How the Bicarbonate Buffer System Responds to pH Changes:
This system's elegance lies in its ability to shift the equilibrium in either direction.
If Blood Becomes Too ACIDIC (Excess H⁺)
The abundant bicarbonate ions (HCO₃⁻) act as proton acceptors, binding to the excess H⁺ to form carbonic acid, a much weaker acid. HCO₃⁻ + H⁺ → H₂CO₃
Respiratory Compensation (Lungs' Role): The carbonic acid formed is unstable and rapidly dissociates back into CO₂ and H₂O. The CO₂ is then exhaled by the lungs. The respiratory system can rapidly increase ventilation (hyperventilation) to "blow off" more CO₂, effectively removing acid from the blood and raising pH.
If Blood Becomes Too BASIC (Too Little H⁺)
The carbonic acid (H₂CO₃) component of the buffer dissociates further, releasing more H⁺ ions into the blood to replenish the deficit. H₂CO₃ → H⁺ + HCO₃⁻
Renal Compensation (Kidneys' Role): The kidneys play a slower but more powerful long-term role. They can excrete excess bicarbonate (HCO₃⁻) if the blood is too basic, or reabsorb more bicarbonate if the blood is too acidic. Crucially, they can also excrete H⁺ directly into the urine and generate "new" bicarbonate ions to be returned to the blood.
Why the Bicarbonate Buffer System is So Effective:
- High Concentration: It is present in high concentrations in the blood.
- Dual Regulation by Organ Systems: The lungs provide rapid control by managing CO₂, while the kidneys provide powerful, long-term control by managing HCO₃⁻.
- Open System: Because CO₂ can be rapidly exhaled, the system is highly adaptable.
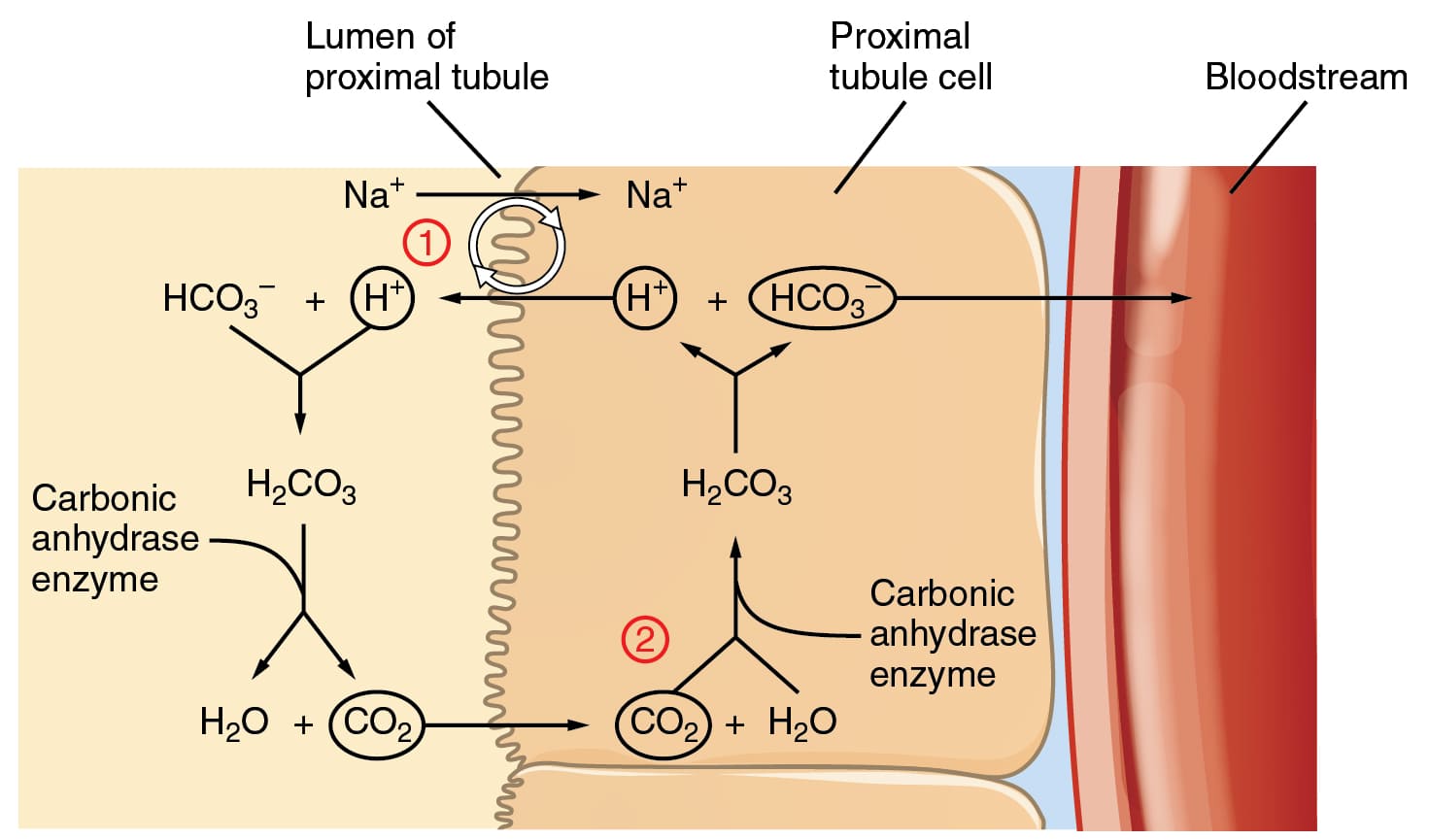
2. The Phosphate Buffer System: Important for Intracellular Fluid and Urine
While less quantitatively significant than the bicarbonate system in the blood plasma, the phosphate buffer system plays a vital and specialized role in the intracellular fluid and the urine.
Phosphate Buffer System Details
Key Locations: It is a crucial intracellular buffer, where phosphate concentrations are high, protecting enzymes and cellular machinery. It is also very important in urine, allowing the kidneys to excrete a significant amount of acid while keeping urine pH within a manageable range.
The Components:
- Weak Acid: Dihydrogen Phosphate (H₂PO₄⁻)
- Conjugate Base: Monohydrogen Phosphate (HPO₄²⁻)
The Dynamic Equilibrium:
H₂PO₄⁻ ⇌ H⁺ + HPO₄²⁻
How it Works:
- If H⁺ Increases (Acidic): The conjugate base (HPO₄²⁻) accepts the excess H⁺ ions to form the weak acid (H₂PO₄⁻).
- If H⁺ Decreases (Basic): The weak acid (H₂PO₄⁻) releases an H⁺ ion to replenish the deficit.
3. The Protein Buffer System: Most Abundant Intracellular Buffer
Proteins are the most abundant macromolecules in the body, accounting for approximately 75% of the body's total buffering capacity. Their power comes from their abundance and the unique chemical groups in their amino acid building blocks.
The Components – Amino Acids and Their Buffering Groups:
- Amino Groups (–NH₂): These are basic groups that can accept H⁺ ions when the environment becomes acidic.
–NH₂ + H⁺ ⇌ –NH₃⁺ - Carboxyl Groups (–COOH): These are acidic groups that can donate H⁺ ions when the environment becomes basic.
–COOH ⇌ –COO⁻ + H⁺
A single large protein molecule can contain many of these groups, allowing it to buffer over a wide range of pH values.
Hemoglobin: A Specialized Protein Buffer
Hemoglobin, the protein in red blood cells, is an exceptionally important buffer, especially for CO₂ transport. As CO₂ from tissues enters red blood cells, it is converted to carbonic acid (H₂CO₃), which then dissociates into H⁺ and HCO₃⁻. Hemoglobin immediately binds to these newly generated H⁺ ions.
The Isohydric Shift: Crucially, deoxygenated hemoglobin (found in the tissues) has a greater affinity for H⁺ than oxygenated hemoglobin. This allows it to efficiently buffer the blood in the tissues where acid is being produced. In the lungs, as hemoglobin picks up oxygen, it releases the H⁺, which recombines with HCO₃⁻ to form CO₂ that is then exhaled. This process is vital for preventing a drastic drop in blood pH during CO₂ transport.
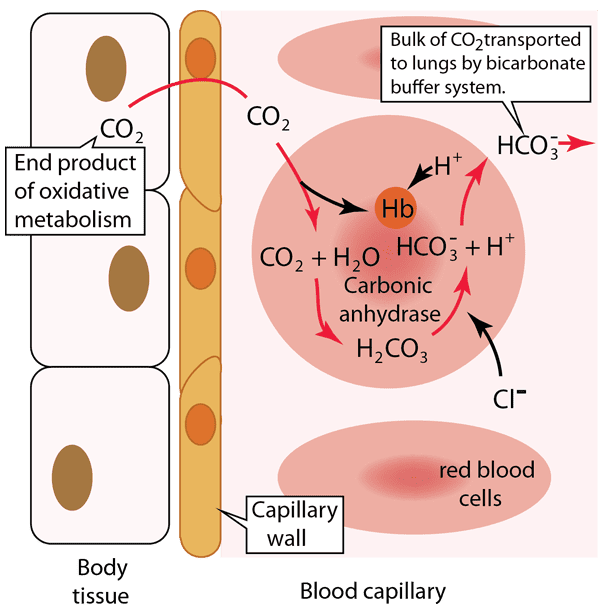
CO₂ Transport and pH Buffering: A Step-by-Step Explanation
Let's break down the critical process of carbon dioxide transport and pH buffering in the blood, a vital concept for medical students. This diagram illustrates what happens in the body tissues and within a blood capillary, focusing on how CO₂ is handled and how the bicarbonate buffer system, aided by hemoglobin, manages the resulting acid.
-
Step 1: Carbon Dioxide Production in Body Tissues
Cellular respiration, the process by which cells generate energy, produces carbon dioxide (CO₂) as a waste product. This newly formed CO₂ quickly diffuses out of the body tissue cells because its concentration is higher inside the cells than in the blood. It then crosses the capillary wall to enter the blood.
-
Step 2: Carbon Dioxide Enters the Red Blood Cell
Once in the blood plasma, a significant portion (about 70-75%) of the CO₂ diffuses into the red blood cells. This is where the magic of the bicarbonate buffer system largely happens for CO₂ transport.
-
Step 3: Formation of Carbonic Acid and Bicarbonate
Inside the red blood cell, the incoming CO₂ immediately reacts with water (H₂O). This reaction is extremely fast due to the presence of a powerful enzyme called carbonic anhydrase. Carbonic anhydrase rapidly catalyzes the conversion of CO₂ and H₂O into carbonic acid (H₂CO₃), which then quickly dissociates (breaks down) into a hydrogen ion (H⁺) and a bicarbonate ion (HCO₃⁻).
-
Step 4: Buffering of Hydrogen Ions by Hemoglobin
The hydrogen ions (H⁺) produced are highly acidic. This is where hemoglobin (Hb), the protein responsible for oxygen transport, plays a crucial buffering role. Hemoglobin readily binds to these H⁺ ions, preventing them from accumulating and causing the blood to become too acidic.
Clinical Relevance for Medical: This step is vital because it explains how the body safely handles the acid generated during CO₂ transport without experiencing a severe drop in blood pH (acidosis) at the tissue level. -
Step 5: Bicarbonate Ion Transport into Plasma (Chloride Shift)
As bicarbonate ions (HCO₃⁻) accumulate inside the red blood cell, they move out into the blood plasma through a special transporter protein. This is how the bulk of CO₂ is transported to the lungs—in the form of HCO₃⁻ in the plasma. To maintain electrical neutrality, as negatively charged HCO₃⁻ ions move out, negatively charged chloride ions (Cl⁻) move into the red blood cell. This exchange is known as the "chloride shift."
Summary of the Overall Process at the Tissue Level:
In the body tissues, CO₂ from metabolism enters red blood cells, where it is rapidly converted to H₂CO₃ and then dissociates into H⁺ and HCO₃⁻. Hemoglobin buffers the H⁺, preventing pH changes, while HCO₃⁻ moves into the plasma (via the chloride shift) to be transported to the lungs. This entire process efficiently removes CO₂ from the tissues and minimizes changes in blood pH.
What happens in the lungs (implied):
When these red blood cells reach the lungs, the process largely reverses. H⁺ detaches from hemoglobin (as hemoglobin binds oxygen), HCO₃⁻ re-enters the red blood cell, recombines with H⁺ to form H₂CO₃, which then rapidly converts back to CO₂ and H₂O. The CO₂ then diffuses out of the red blood cell and into the alveoli of the lungs to be exhaled.
How These Systems Work Together to Maintain Homeostasis
These buffer systems collaborate in a multi-tiered defense strategy:
First Line of Defense (Rapid & Immediate): Chemical Buffer Systems
The bicarbonate, phosphate, and protein buffer systems provide immediate buffering within milliseconds to seconds. They are always active, chemically neutralizing any H⁺ excess or deficit to "absorb the shock" and buy time for the physiological systems to respond.
Second Line of Defense (Intermediate): The Lungs
The respiratory system acts as a rapid-response physiological buffer, responding within minutes to hours. The lungs can quickly adjust the rate of breathing:
- Hyperventilation (increased breathing) blows off more CO₂, effectively removing carbonic acid from the blood to increase pH (correct acidosis).
- Hypoventilation (decreased breathing) retains CO₂, increasing carbonic acid to decrease pH (correct alkalosis).
Third Line of Defense (Long-Term): The Kidneys
The kidneys are the most powerful and precise regulators of pH, though they act more slowly (hours to days). They are responsible for the long-term maintenance of acid-base balance by:
- Excreting or reabsorbing bicarbonate ions (HCO₃⁻) as needed.
- Excreting excess H⁺ into the urine, often buffered by phosphate and ammonia.
- Generating new bicarbonate ions, a critical function, especially in chronic acidosis.
Biochemistry Lesson Three
Acids, Bases, and Buffers
Test your knowledge with these 20 questions.
Biochemistry: Acids & Bases
Question 1/20
Quiz Complete!
Here are your results, .
Your Score
18/20
90%