Renal Clearance & Micturition
Renal Clearance
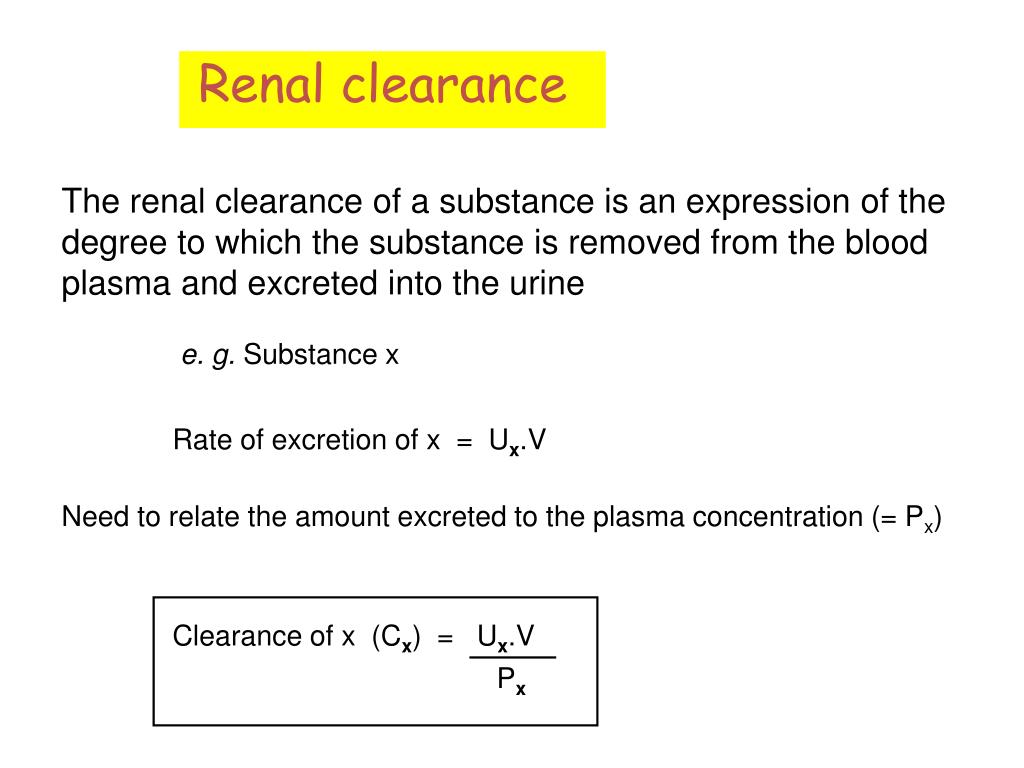
Clearance is a quantitative measure of how effectively the kidneys remove a particular substance from the blood plasma.
It represents the hypothetical volume of plasma that would be completely cleared of a substance per unit of time.
Cx = (Ux * V) / Px
- Cx = Renal clearance of substance X (in mL/min or mL/s)
- Ux = Concentration of substance X in urine (e.g., mg/dL or mg/mL)
- V = Urine flow rate (e.g., mL/min)
- Px = Concentration of substance X in plasma (e.g., mg/dL or mg/mL)
Interpretation of the Formula:
- (Ux * V) represents the excretion rate of substance X – the total amount of X removed from the body via urine per minute.
- Px represents the concentration of X in the "incoming" plasma.
- Thus, clearance essentially asks: "What volume of plasma must have been 'purified' to account for the amount of substance X excreted in the urine?"
Relationship to Renal Handling: The amount of substance excreted is a net result of three processes:
Excretion Rate = Filtration Rate - Reabsorption Rate + Secretion Rate
Ux * V = (GFR * Px) - T_reabsorption + T_secretion
- GFR = Glomerular Filtration Rate
- T_reabsorption = Tubular reabsorption rate
- T_secretion = Tubular secretion rate
Importance of Renal Clearance
Renal clearance measurements are invaluable tools for assessing various aspects of renal function:
- Quantifying Glomerular Filtration Rate (GFR): The gold standard for measuring kidney function.
- Estimating Renal Plasma Flow (RPF): Gives insight into blood supply to the kidneys.
- Assessing Severity of Renal Damage: Decreased GFR and RPF can indicate kidney disease progression.
- Characterizing Tubular Reabsorption: By comparing a substance's clearance to GFR, we can determine if it's reabsorbed.
- Characterizing Tubular Secretion: Similarly, by comparison to GFR, we can determine if a substance is secreted.
Clearance Tests: Endogenous vs. Exogenous Markers
Endogenous Markers
Substances naturally produced by the body.
- Creatinine: Clinically most common for GFR estimation.
- Urea: Not a good GFR marker due to significant reabsorption.
- Uric Acid: Significant reabsorption and secretion.
Exogenous Markers
Substances administered externally for diagnostic purposes.
- Inulin: The gold standard for GFR research.
- Para-aminohippuric acid (PAH): Gold standard for RPF measurement.
- Diodrast: Similar properties to PAH, historically used for RPF.
Measurement of Glomerular Filtration Rate (GFR)
GFR is the volume of fluid filtered from the glomerular capillaries into Bowman's capsule per unit time. It's the best overall index of kidney function.
Criteria for an Ideal GFR Marker:
An ideal substance for measuring GFR must possess the following characteristics:
- Freely Filtered: It must pass unimpeded across the glomerular filtration barrier.
- Not Reabsorbed: No reabsorption from the renal tubules back into the blood.
- Not Secreted: No secretion from the blood into the renal tubules.
- Not Metabolized: It should not be broken down by the kidneys or other tissues.
- Not Stored: Should not accumulate in the body.
- Not Protein Bound: If bound, only the free fraction is filtered.
- Physiologically Inert/Non-toxic: Should not affect renal function or be harmful.
- Easily Measured: Detectable in plasma and urine with reliable assays.
1. Inulin Clearance: The Gold Standard for Research GFR
- Properties: Perfectly fits all criteria for an ideal GFR marker. It is a polysaccharide, freely filtered, and neither reabsorbed nor secreted.
- Method: Requires continuous intravenous infusion to maintain a steady plasma concentration. Urine is collected over a timed period.
- Limitation: It is exogenous and requires continuous infusion, making it impractical for routine clinical use.
Calculation Example:
Assume:
- [inulin]urine = 30mg/ml
- [inulin]plasma = 0.5mg/ml
- Urine flow rate = 2ml/ml
GFR = 120ml/min
2. Creatinine Clearance: The Clinical Standard for GFR Estimation
- Properties:
- Endogenously produced by muscle metabolism at a relatively constant rate.
- Freely filtered at the glomerulus.
- A small amount is secreted by the proximal tubule (error 1: amount excreted > amount filtered). This means creatinine clearance slightly overestimates true GFR.
- Analytical Interference: Older spectrophotometric methods (e.g., Jaffe reaction) detect chromogens other than true creatinine, leading to an overestimation of plasma creatinine concentration (error 2).
- Clinical Utility:
- Convenience: Does not require intravenous infusion. Can be estimated from a 24-hour urine collection or, more commonly, estimated from serum creatinine using prediction equations (e.g., Cockcroft-Gault, MDRD, CKD-EPI).
- Fortuitous Cancellation of Errors: In healthy individuals, the overestimation of GFR due to secretion is often roughly canceled out by the overestimation of plasma creatinine by older assays. However, this balance is disturbed in kidney disease, extreme muscle mass, or with certain medications.
General Principle: Relating Clearance to Renal Handling
The comparison of a substance's clearance (Cx) with the GFR (measured by Cinulin or estimated by Ccreatinine) provides insight into how the kidney handles that substance:
- If Cx = Cinulin (or GFR): The substance is only filtered (not reabsorbed, not secreted).
- Example: Inulin.
- If Cx < Cinulin (or GFR): The substance is filtered and net reabsorbed by the renal tubules.
- The kidneys remove less of the substance from the plasma than the volume of plasma filtered.
- Example: Glucose (normally 100% reabsorbed, so clearance is 0 unless plasma glucose exceeds tubular maximum), sodium, urea.
- Note: If a substance is completely reabsorbed (e.g., glucose at normal plasma levels), its clearance is effectively 0. If Ux * V = 0, then Cx = 0.
- If Cx > Cinulin (or GFR): The substance is filtered and net secreted by the renal tubules.
- The kidneys remove more of the substance from the plasma than the volume of plasma filtered. This indicates that the tubules are actively adding the substance to the urine.
- Example: PAH, creatinine (to a small extent).
Measurement of Renal Plasma Flow (RPF)
RPF is the volume of plasma flowing through the kidneys per unit time.
Ideal RPF Marker Criteria: An ideal substance for measuring RPF must be:
- Freely Filtered.
- Completely Secreted: All of the substance that enters the renal artery (both filtered and non-filtered) must be removed by either filtration or tubular secretion in a single pass through the kidney.
- Not Reabsorbed.
- Not Metabolized or Stored.
- Physiologically Inert/Non-toxic.
- Easily Measured.
Para-aminohippuric acid (PAH) Clearance: The Gold Standard for RPF
- Properties: PAH is the prototypical substance for measuring effective RPF (ERPF).
- It is freely filtered.
- It is actively secreted by the proximal tubules.
- At low plasma concentrations, virtually all PAH delivered to the kidneys (both filtered and non-filtered plasma) is removed in one pass. Approximately 90% is extracted from the plasma; therefore, PAH clearance provides a good estimate of effective RPF (ERPF).
- Method: Requires intravenous infusion.
- Logic: If all PAH entering the kidney in the renal artery plasma is excreted in the urine, then the volume of plasma containing that amount of PAH must be the RPF.
- Amount of PAH entering the kidneys per minute = PPAH * RPF
- Amount of PAH excreted per minute = UPAH * V
- Since these amounts are equal: PPAH * RPF = UPAH * V
- Therefore: RPF = (UPAH * V) / PPAH
- Hence RPF = Clearance of PAH
- UPAH = 25.2 mg/ml (conc. Of PAH in urine)
- V = 1.1 ml/min (Urine flow)
- PPAH = 0.05 mg/ml (conc. Of PAH in blood)
Then CPAH = (25.2 X 1.1) / 0.05 = 560ml/min
Renal Blood Flow (RBF) Calculation
Once RPF is known, total Renal Blood Flow (RBF) can be calculated using the hematocrit (Hct), which is the percentage of red blood cells in the blood.
RBF = RPF / (1 - Hct)
This means approximately 1 liter of blood flows through the kidneys per minute, highlighting their immense perfusion.
Filtration Fraction (FF)
The filtration fraction is the proportion of the renal plasma flow that is filtered at the glomerulus.
FF = GFR / RPF
- Normal Value: Approximately 0.20 (20%), meaning about 20% of the plasma entering the glomeruli is filtered.
- Clinical Significance: Changes in FF can indicate alterations in glomerular or tubular function. For example, increased FF can occur with efferent arteriolar constriction.
Micturition
Micturition (also known as voiding, urination, or uresis) is the physiological process of expelling urine from the urinary bladder through the urethra and out of the body.
In healthy adults, this is a coordinated process under voluntary control, while in infants or individuals with neurological impairment, it can occur as an involuntary reflex.
I. Physiological Anatomy of the Lower Urinary Tract
Understanding the structures involved is crucial for grasping the mechanics of micturition:
Kidneys & Ureters
- Kidneys: Urine production occurs here.
- Ureters: Muscular tubes (smooth muscle arranged in spiral, longitudinal, and circular bundles) that transport urine from the renal pelvis to the urinary bladder.
- Peristaltic waves (occurring 1-5 times/minute) are initiated in the renal pelvis by increasing pressure from accumulating urine. These waves propel urine towards the bladder.
- Ureterovesical Junction: The ureters enter the bladder wall obliquely, creating a flap-valve mechanism. This prevents the backflow (reflux) of urine from the bladder into the ureters, especially during bladder contraction.
- Vesicoureteral Reflux: If the length of the ureter within the bladder wall is too short, this valve can be incompetent, leading to urine flowing backward into the ureters, which can cause kidney infections.
- Ureterorenal Reflex: A crucial reflex. Severe pain (e.g., from a ureteral stone) triggers intense ureteral constriction. Pain signals also elicit a sympathetic reflex that constricts renal arterioles, reducing blood flow and urine formation in the affected kidney, thus reducing pressure behind the obstruction.
Urinary Bladder
A distensible, muscular sac designed for urine storage.
- Body: The main storage portion of the bladder.
- Neck: The funnel-shaped inferior part that connects to the urethra.
- Detrusor Muscle: The main smooth muscle of the bladder wall. It's composed of intertwining muscle fibers, and its contraction is responsible for emptying the bladder. It maintains low pressure during filling (compliance) and generates high pressure during voiding.
- Trigone: A smooth, triangular region on the internal posterior wall of the bladder. It's bordered by the two ureteral openings and the internal urethral orifice. It's less distensible than the rest of the bladder.
Urethra & Sphincters
Urethra: A tube that carries urine from the bladder to the outside of the body.
Sphincters: Crucial for continence.
- Internal Urethral Sphincter: Located at the bladder neck. It is composed of smooth muscle and is under involuntary (autonomic) control. It is functionally a thickening of the detrusor muscle. In males, it also helps prevent semen reflux into the bladder during ejaculation.
- External Urethral Sphincter: Located in the urogenital diaphragm, distal to the internal sphincter. It is composed of skeletal muscle and is under voluntary (somatic) control.
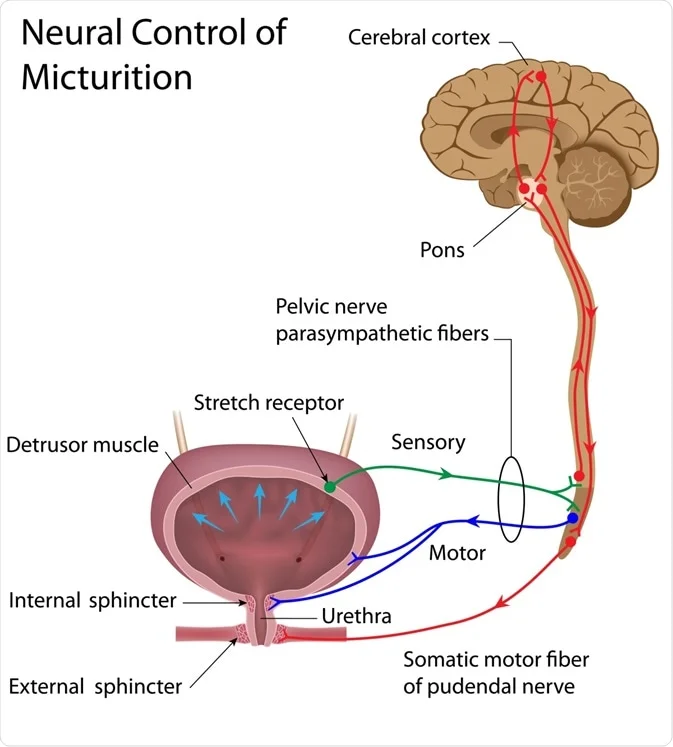
II. Innervation of the Bladder and Urethra
The lower urinary tract is innervated by a complex interplay of the autonomic and somatic nervous systems.
1. Parasympathetic Nerves
(Pelvic Nerves - S2-S4 Spinal Cord Segments)
- Sensory (Afferent): Carry stretch (mechanoreceptor) signals from the detrusor muscle to the spinal cord, indicating bladder filling. These are crucial for initiating the micturition reflex.
- Motor (Efferent): Excitatory to the detrusor muscle, causing it to contract, and inhibitory to the internal urethral sphincter (causing it to relax). This promotes bladder emptying. Note: Some texts state the internal sphincter is primarily regulated by sympathetic input during storage and parasympathetic inhibition during voiding, or simply that it relaxes passively as the detrusor contracts via its physical connection.
2. Sympathetic Nerves
(Hypogastric Nerves - L1-L3 Spinal Cord Segments)
- Sensory (Afferent): Primarily transmit signals related to pain and overdistension (extreme fullness) from the bladder.
- Motor (Efferent):
- Relax the detrusor muscle (via beta-3 adrenergic receptors) during bladder filling to allow for storage at low pressure.
- Contract the internal urethral sphincter (via alpha-1 adrenergic receptors) to maintain continence.
- Innervate blood vessels in the bladder.
- Role in Ejaculation: Sympathetic activity causes contraction of the internal sphincter to prevent retrograde ejaculation into the bladder.
3. Somatic Nerves
(Pudendal Nerves - S2-S4 Spinal Cord Segments)
- Sensory (Afferent): Carry sensory information from the urethra and external urethral sphincter, contributing to awareness of bladder fullness and the urge to void.
- Motor (Efferent): Excitatory to the external urethral sphincter, allowing for voluntary contraction to maintain continence or voluntary relaxation to initiate voiding. This is the voluntary control component.
III. The Micturition Reflex: Storage and Voiding Phases
The process of micturition is a coordinated reflex primarily involving the spinal cord, but it is heavily modulated by higher brain centers.
A. Storage Phase (Bladder Filling):
- Low Intravesical Pressure: The detrusor muscle is highly compliant, meaning it can stretch significantly without a large increase in internal pressure (due to its viscoelastic properties and sympathetic inhibition).
- Continence Maintained:
- Internal Sphincter: Tonically contracted due to sympathetic stimulation.
- External Sphincter: Tonically contracted due to continuous somatic innervation via the pudendal nerve (voluntary control).
- Afferent Signals: As the bladder fills, stretch receptors in the detrusor muscle send increasing signals via the pelvic nerves to the sacral spinal cord. These signals also ascend to the brain (pons, cerebral cortex) to create the sensation of bladder fullness.
- Sympathetic Dominance: During filling, the sympathetic nervous system is dominant, promoting detrusor relaxation and internal sphincter contraction.
B. Voiding Phase (Micturition Reflex):
When bladder volume reaches a threshold (typically 150-300 ml for a conscious urge, 300-400 ml for a strong urge):
- Afferent Sensory Signals Intensify: Strong stretch signals from the bladder wall ascend to the pontine micturition center (PMC) in the brainstem and the cerebral cortex.
- Voluntary Decision to Void:
- If appropriate to void, the cerebral cortex sends inhibitory signals to the external urethral sphincter (relaxing it) and excitatory signals to the pontine micturition center.
- If not appropriate, the cortex sends inhibitory signals to the PMC and reinforces external sphincter contraction.
- Activation of the Pontine Micturition Center (PMC): The PMC acts as a "switch." Once activated, it:
- Inhibits sympathetic outflow to the bladder (stopping detrusor relaxation and internal sphincter contraction).
- Activates parasympathetic outflow to the bladder via the pelvic nerves (causing detrusor contraction and internal sphincter relaxation).
- Inhibits somatic outflow to the external urethral sphincter via the pudendal nerves (causing its relaxation).
- Detrusor Contraction: The bladder contracts forcefully, increasing intravesical pressure.
- Sphincter Relaxation: Both internal (involuntarily) and external (voluntarily) sphincters relax.
- Urine Expulsion: Urine is expelled through the urethra.
- Reflex Termination: Once the bladder is empty, stretch receptor activity ceases, leading to the inhibition of the PMC and the return to the storage phase (sympathetic dominance and sphincter contraction).
IV. Brain Centers Regulating Micturition
- Spinal Cord (Sacral Micturition Center): The basic reflex arc is located here. It can operate autonomously in infants or in cases of spinal cord injury above the sacral segments, leading to an involuntary reflex bladder.
- Pontine Micturition Center (PMC, "Bartholomew's Nucleus"): Located in the brainstem. This is the primary coordinating center for the micturition reflex. It orchestrates the synergistic relaxation of the sphincters and contraction of the detrusor during voiding.
- Periaqueductal Gray (PAG): A midbrain structure that receives sensory input from the bladder and relays it to the PMC and cortex. It plays a role in the urge to void and emotional modulation of micturition.
- Cerebral Cortex (Frontal Lobe, Insula, Cingulate Gyrus): Provides voluntary control over the micturition reflex. It can override or initiate the reflex based on social appropriateness and personal will. Damage to these areas can lead to urinary incontinence (e.g., in stroke or dementia).
Modulation by Higher Centers (Voluntary Control)
As the nervous system matures, higher brain centers gain significant control over the basic micturition reflex. This allows for socially appropriate timing of urination.
Role of the Pons (Pontine Micturition Center - PMC):
- The PMC, located in the brainstem, is a major relay center and coordinates the entire voiding act.
- During Bladder Filling: Stretch receptor signals ascend from the spinal cord to the pons and then to the brain (cerebral cortex). This creates the perception of bladder fullness and the desire to urinate.
- Inhibition to Postpone Voiding: Normally, the brain sends inhibitory signals to the PMC to prevent it from activating the micturition reflex. This keeps the detrusor relaxed and the sphincters contracted, allowing urine storage even with a strong urge.
- Activation to Initiate Voiding: When it is timely and appropriate to urinate, the brain removes its inhibition from the PMC and sends excitatory signals. The PMC then orchestrates voiding by:
- Activating parasympathetic outflow to the detrusor and internal sphincter.
- Inhibiting somatic outflow to the external urethral sphincter.
- Coordination: The PMC ensures the coordinated relaxation of the urethral sphincters and contraction of the detrusor – a crucial synergy for effective voiding.
Role of the Cerebral Cortex (Frontal Lobe):
- The cerebral cortex (especially the frontal lobe) provides the ultimate voluntary control.
- It receives sensory input regarding bladder fullness.
- It sends tonically inhibitory signals to the detrusor muscle (via the PMC) to prevent premature emptying.
- It also controls the external urethral sphincter via the pudendal nerve (somatic innervation), allowing for voluntary contraction (to hold urine) or relaxation (to initiate voiding).
- The cortical centers weigh social appropriateness and personal control to decide when to allow micturition.
Micturition Reflex
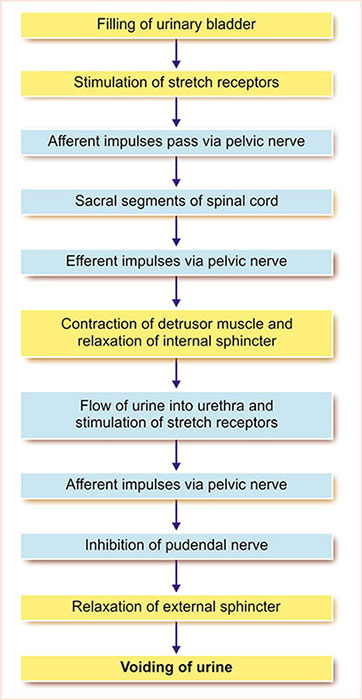
- Filling of the urinary bladder
- Stimulation of stretch receptors
- Afferent impulses pass through the pelvic nerve and reach the spinal cord
- Efferent impulses through the pelvic nerve
- Contraction of the detrusor muscle and relaxation of the internal sphincter
- The flow of urine into the urethra and stimulation of stretch receptors
- Afferent impulses through the pelvic nerve
- Inhibition of pudendal nerve
- Relaxation of the external sphincter
- Voiding of the urine or micturition
The micturition reflex is the spinal cord reflex that leads to bladder emptying. While it can function autonomously (as in infants or after certain neurological injuries), it is normally under significant control and modulation by higher brain centers, allowing for voluntary initiation or inhibition.
Basic Micturition Reflex (Spinal Cord Level)
This reflex forms the fundamental mechanism for bladder emptying. It is centered in the sacral spinal cord (S2, S3, S4 segments).
- Stimulus: As the bladder fills with urine (typically 300-400 ml, though the first desire to urinate may be around 150-200 ml), stretch receptors in the detrusor muscle of the bladder wall are activated.
- Afferent Pathway: Sensory nerve impulses travel from these stretch receptors via the pelvic nerves (parasympathetic afferents) to the sacral spinal cord.
- Integration Center: The sacral spinal cord serves as the integration center for this reflex.
- Efferent Pathway (Parasympathetic): Motor impulses are conducted through parasympathetic fibers of the pelvic nerves back to the bladder.
- Effector Response:
- Detrusor muscle contracts: The efferent signals excite the detrusor muscle, causing it to contract.
- Internal Urethral Sphincter relaxes: The same parasympathetic signals (or inhibition of sympathetic tone) cause the involuntary internal urethral sphincter to relax.
Outcome in Infants (Primitive Voiding Center): In infants and young children, whose brain development has not yet established full voluntary control, this spinal reflex predominates. Bladder filling automatically triggers detrusor contraction and sphincter relaxation, leading to involuntary voiding.
The Bladder Filling & Emptying Cycle:
- Bladder Fills: Urine enters the bladder via the ureters. The detrusor muscle relaxes (accommodates), and both internal and external sphincters contract to maintain continence.
- First Desire to Urinate: (e.g., bladder half full, ~150-200 ml) Stretch receptors send signals to the brain, but the cortical inhibition of the PMC and active contraction of the external sphincter prevent voiding.
- Urination Voluntarily Inhibited: Cortical control keeps the external sphincter contracted and inhibits the PMC, thus keeping the detrusor relaxed.
- Appropriate Time to Void:
- The brain removes inhibition from the PMC and activates it.
- PMC stimulates parasympathetic nerves to the bladder: detrusor contracts, internal sphincter relaxes.
- PMC inhibits somatic nerves to the external sphincter: external sphincter relaxes.
- Voluntary contraction of abdominal muscles can aid in increasing voiding pressure.
- Voiding: Urine is expelled.
- After Voiding: Detrusor relaxes, sphincters close, and the cycle restarts.
Phases of Micturition:
When the micturition reflex is activated and permitted:
- Progressive and Rapid Increase in Pressure: Detrusor contraction causes a sharp rise in intravesical pressure.
- Period of Sustained Pressure: The detrusor maintains contraction, leading to sustained high pressure, facilitating urine expulsion.
- Return of Pressure to Basal Tone: Once the bladder is largely empty, the detrusor relaxes, and pressure returns to the resting (basal) tone.
Abnormalities of Micturition (Neurogenic Bladder Conditions)
Damage to different parts of the nervous system can lead to various forms of neurogenic bladder:
1. Atonic Bladder (Sensory Denervation)
- Cause: Destruction of sensory nerve fibers from the bladder to the spinal cord (e.g., crush injury, syphilis affecting dorsal roots).
- Effect: No sensory input means the person doesn't feel bladder fullness. The bladder becomes flaccid, overfilled, and non-contractile.
- Outcome: Overflow incontinence (urine dribbles out when intravesical pressure exceeds urethral resistance).
2. Automatic Bladder (Spastic Neurogenic Bladder/Upper Motor Neuron Lesion)
- Cause: Complete transection of the spinal cord above the sacral region, but with intact sacral cord segments and peripheral nerves to the bladder.
- Effect: The bladder loses all communication with higher brain centers. The basic sacral micturition reflex is intact but uninhibited.
- Outcome: After an initial phase of spinal shock (where the bladder is flaccid and unresponsive), the sacral reflex returns. The bladder will then empty automatically and completely whenever a certain volume of urine accumulates, without voluntary control. This is often characterized by frequent, small volume voiding.
3. Uninhibited Neurogenic Bladder
- Cause: Partial damage in the spinal cord or brainstem, interrupting some inhibitory signals from higher centers.
- Effect: The micturition reflex becomes highly active.
- Outcome: Even a slight quantity of urine can elicit an uncontrollable and frequent micturition reflex, leading to urgency and sometimes incontinence.
4. Nocturnal Micturition (Bed Wetting/Enuresis)
- In Children: Normal in infants and children below 3-5 years due to incomplete myelination of motor nerve fibers and insufficient maturation of cortical control over the micturition reflex during sleep.
- In Adults: Can indicate an underlying medical condition.
5. Incontinence from Impaired Sphincter Function
- Cause: Weakness or damage to the external urethral sphincter (e.g., after multiple childbirths in women, prostatic surgery in men, or aging).
- Outcome: Urine leakage, especially in response to sudden increases in intra-abdominal/intravesical pressure (e.g., coughing, sneezing, laughing, lifting) – known as stress incontinence.
Questions (Qns)
- Peristaltic contractions in the ureter are enhanced by sympathetic stimulation: a. T b. F
- The micturition reflex is centered in the: A. Medulla B. Sacral cord C. Hypothalamus D. Lumbar cord
- Which of these is under voluntary control: A. Urethra B. Detrusor muscle C. Internal sphincter D. External sphincter
- Which of the following actions happen when the sympathetic is activated: A. bladder contraction, sphincter relaxation B. bladder relaxation, sphincter contraction
- A person had a car accident and there was an injury in his spinal cord(L1,L2) after the initial phase of spinal shock, what happened to the bladder? A. paralyzed and flaccid B. Emptying with voluntary control C. Loss of voluntary control
Answers to Practice Questions:
1. Peristaltic contractions in the ureter are enhanced by sympathetic stimulation:
b. F (False) - Ureteral peristalsis is largely intrinsic to the ureteric smooth muscle, influenced by stretch. While autonomic nerves modulate it, sympathetic stimulation tends to decrease activity, while parasympathetic increases it. The ureterorenal reflex (sympathetic constriction of renal arterioles) is a different mechanism.
2. The micturition reflex is centered in the:
B. Sacral cord - This is the primary spinal cord reflex center.
3. Which of these is under voluntary control:
D. External sphincter - Composed of skeletal muscle, it is consciously controlled via the pudendal nerve.
4. Which of the following actions happen when the sympathetic is activated:
B. bladder relaxation, sphincter contraction - Sympathetic activity promotes urine storage by relaxing the detrusor (beta-3 receptors) and contracting the internal sphincter (alpha-1 receptors).
5. A person had a car accident and there was an injury in his spinal cord (L1, L2) after the initial phase of spinal shock, what happened to the bladder?
C. Loss of voluntary control (and it would become an automatic bladder, emptying reflexively). An injury at L1/L2 means the sacral cord and its connections to the bladder are intact, but connections to higher brain centers are cut. Therefore, the bladder would eventually become "automatic" after spinal shock.
https://www.doctorsrevisionuganda.com | Whatsapp: 0726113908
Renal Clearance & Micturition Quiz
Systems Physiology
Enter your details to begin the examination.
🛡️ Privacy Note: Results are for tracking and certification purposes only.
Renal Clearance & Micturition Quiz
Systems Physiology
Preparing questions...
Exam Completed!
See your performance breakdown below.