Pentose Phosphate Pathway: PPP
Pentose Phosphate Pathway (PPP)
The Pentose Phosphate Pathway (PPP), also known as the Hexose Monophosphate Shunt (HMP Shunt), is an alternative metabolic route for glucose metabolism that runs parallel to glycolysis. The HMP pathway is also known as the Warburg-Dickens pathway. About 10% of glucose entering this pathway per day. The liver & RBCs metabolise about 30% of glucose by this pathway.
Unlike glycolysis, its primary purpose is not to generate ATP. Instead, its main functions are:
- Production of NADPH: Essential for reductive biosynthetic reactions and for protecting cells from oxidative stress.
- Production of Ribose-5-Phosphate: A vital precursor for the synthesis of nucleotides (DNA, RNA) and coenzymes.
Think of the PPP as a "shunt" because it diverts glucose-6-phosphate away from glycolysis to serve these distinct purposes, and can then feed intermediates back into glycolysis. It primarily occurs in the cytosol of cells.
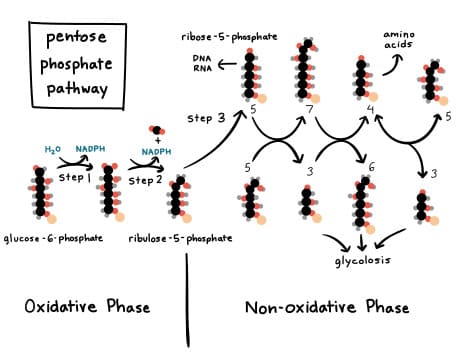
Two Major Phases of the PPP
The Pentose Phosphate Pathway is divided into two distinct phases:
a) The Oxidative (Irreversible) Phase:
- Function: This phase is responsible for the generation of NADPH and the production of ribulose-5-phosphate (which is then converted to ribose-5-phosphate).
- Nature: It is largely irreversible.
- Key Reactions: Involves oxidative decarboxylation reactions where glucose-6-phosphate is oxidized, releasing CO₂, and reducing NADP⁺ to NADPH.
b) The Non-Oxidative (Reversible) Phase:
- Function: This phase interconverts various sugar phosphates, primarily transforming pentose phosphates into glycolytic intermediates (fructose-6-phosphate and glyceraldehyde-3-phosphate). This allows carbon skeletons to be recycled back into glycolysis or used for gluconeogenesis.
- Nature: This phase is entirely reversible.
- Key Enzymes: Involves transketolase and transaldolase enzymes, which facilitate the transfer of two-carbon and three-carbon units, respectively.
Products of the PPP
The PPP is critically important because it provides two essential molecules:
a) NADPH (Nicotinamide Adenine Dinucleotide Phosphate, reduced form)
- Structure: Similar to NADH, but with an additional phosphate group.
- Function: Unlike NADH (used in catabolism for ATP), NADPH is predominantly used in anabolic (biosynthetic) processes and as a reductant in antioxidant defense.
- Reductive Biosynthesis: Providing reducing power for the synthesis of fatty acids, cholesterol, and steroid hormones. Tissues actively involved in these syntheses (e.g., liver, adipose tissue, adrenal cortex) have a highly active PPP.
- Antioxidant Defense: Protecting cells from damage by reactive oxygen species (ROS) by maintaining the reduced state of glutathione.
b) Ribose-5-Phosphate
- Structure: A five-carbon sugar phosphate.
- Function: This molecule is the direct precursor for the synthesis of:
- Nucleotides: The building blocks of DNA and RNA.
- Coenzymes: Such as ATP, NADH, FADH₂, and Coenzyme A.
- Demand: Cells that are rapidly dividing (e.g., bone marrow, skin, cancer cells) will have a high demand for ribose-5-phosphate.
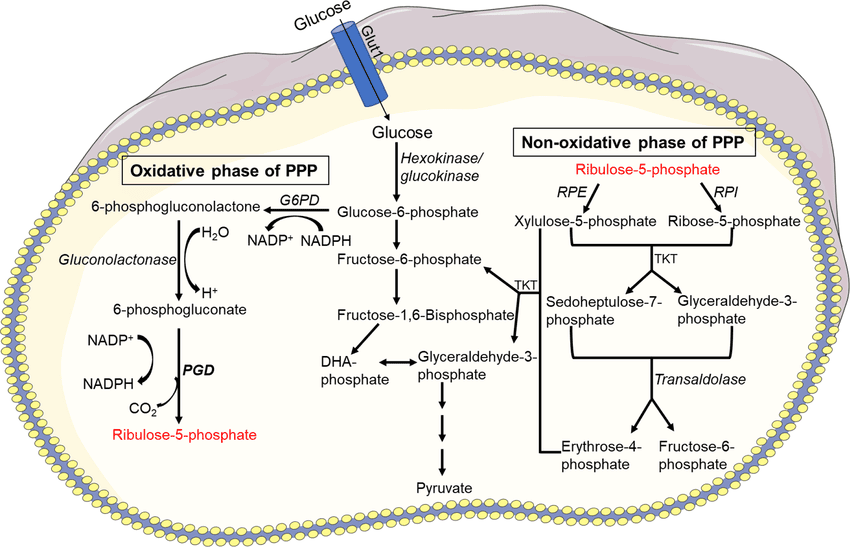
Location of the pathway
- The enzymes are located in the cytosol.
- The tissues such as liver, adipose tissue, adrenal gland, erythrocytes, testes & lactating mammary gland, are highly active in the HMP shunt.
- Most of these tissues are involved in the biosynthesis of fatty acids and steroids, which are dependent on the supply of NADPH.
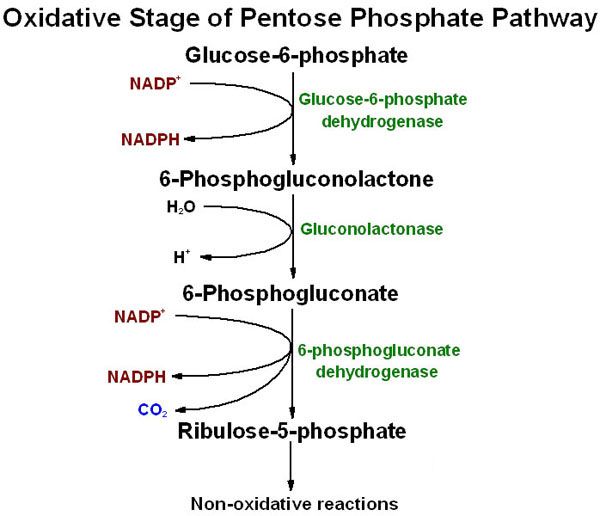
The Oxidative (Irreversible) Phase
This phase consists of three main reactions, starting with glucose-6-phosphate and culminating in the production of NADPH and ribulose-5-phosphate.
Key Concepts of the Oxidative Phase:
- Irreversible: The reactions in this phase are essentially unidirectional under physiological conditions.
- NADPH Production: This is the primary site of NADPH generation. Each molecule of glucose-6-phosphate entering this phase yields two molecules of NADPH.
- Substrate: Glucose-6-phosphate, which is also an intermediate in glycolysis.
- Location: Occurs in the cytosol.
The Three Reactions of the Oxidative Phase:
The oxidative phase involves the following sequential reactions:
1. Glucose-6-Phosphate Dehydrogenation (The Rate-Limiting Step)
- Enzyme: Glucose-6-Phosphate Dehydrogenase (G6PD)
- Reaction: Glucose-6-phosphate is oxidized, and NADP⁺ is reduced to NADPH. A lactone (cyclic ester) intermediate, 6-phosphogluconolactone, is formed.
- Equation:
Glucose-6-phosphate + NADP⁺ → 6-Phosphogluconolactone + NADPH + H⁺ - Significance: This is the rate-limiting and committed step of the entire Pentose Phosphate Pathway. The activity of G6PD is highly regulated.
2. Hydrolysis of 6-Phosphogluconolactone
- Enzyme: 6-Phosphogluconolactonase
- Reaction: The lactone ring is hydrolyzed to an open-chain carboxylic acid, 6-phosphogluconate.
- Equation:
6-Phosphogluconolactone + H₂O → 6-Phosphogluconate - Significance: This step prepares the molecule for the second oxidative reaction.
3. Oxidative Decarboxylation of 6-Phosphogluconate
- Enzyme: 6-Phosphogluconate Dehydrogenase
- Reaction: 6-phosphogluconate undergoes oxidative decarboxylation, meaning it is oxidized (another molecule of NADP⁺ is reduced to NADPH) and a molecule of CO₂ is released. The product is Ribulose-5-phosphate.
- Equation:
6-Phosphogluconate + NADP⁺ → Ribulose-5-phosphate + NADPH + H⁺ + CO₂ - Significance: This reaction generates the second molecule of NADPH and the first pentose phosphate, which serves as the entry point into the non-oxidative phase.
Summary of the Oxidative Phase:
The net reaction for the oxidative phase is:
→
Ribulose-5-phosphate + 2 NADPH + 2 H⁺ + CO₂
Key Takeaways from the Oxidative Phase:
- Two molecules of NADPH are produced per molecule of glucose-6-phosphate.
- One molecule of CO₂ is released.
- Ribulose-5-phosphate (a pentose sugar) is the final product.
- G6PD is the critical, rate-limiting enzyme.
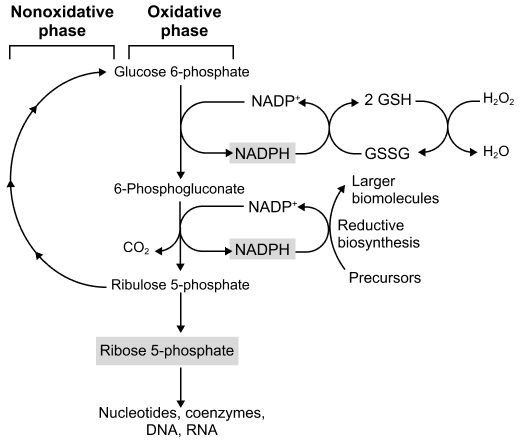
The Non-Oxidative (Reversible) Phase
The non-oxidative phase is a series of reversible reactions that interconvert various sugar phosphates. Its primary functions are:
- Conversion of Ribulose-5-Phosphate: To other pentose phosphates, including ribose-5-phosphate (essential for nucleotide synthesis).
- Recycling of Carbon Skeletons: To produce glycolytic intermediates (fructose-6-phosphate and glyceraldehyde-3-phosphate) from excess pentose phosphates, linking the PPP back to glycolysis.
- Flexibility: The reversibility allows the cell to adjust the production of ribose-5-phosphate and NADPH according to its needs.
Key Enzymes and Reactions of the Non-Oxidative Phase
This phase involves three main enzymes: an isomerase, an epimerase, and two transketolases/transaldolases.
1. Interconversion of Pentose Phosphates
The ribulose-5-phosphate generated in the oxidative phase needs to be converted into other pentose sugars.
a) Ribulose-5-Phosphate Isomerase
- Enzyme: Ribose-5-phosphate Isomerase
- Reaction: Converts the ketose sugar ribulose-5-phosphate into the aldose sugar ribose-5-phosphate. This is crucial as ribose-5-phosphate is the direct precursor for nucleotide synthesis.
- Equation:
Ribulose-5-phosphate ⇌ Ribose-5-phosphate
b) Ribulose-5-Phosphate Epimerase
- Enzyme: Xylulose-5-phosphate Epimerase
- Reaction: Converts ribulose-5-phosphate into another ketose sugar, xylulose-5-phosphate, which is important for subsequent transketolase reactions.
- Equation:
Ribulose-5-phosphate ⇌ Xylulose-5-phosphate
2. Transketolase and Transaldolase Reactions (The "Shunt" Part)
These two enzymes are responsible for moving two-carbon and three-carbon units between sugar phosphates to produce glycolytic intermediates.
a) Transketolase
- Enzyme: Transketolase
- Cofactor: Requires Thiamine Pyrophosphate (TPP).
- Function: Transfers a two-carbon (ketol) unit.
- First Reaction: Transfers 2 carbons from Xylulose-5-phosphate (5C) to Ribose-5-phosphate (5C), producing Glyceraldehyde-3-phosphate (3C) and Sedoheptulose-7-phosphate (7C).
b) Transaldolase
- Enzyme: Transaldolase
- Function: Transfers a three-carbon unit.
- Reaction: Transfers 3 carbons from Sedoheptulose-7-phosphate (7C) to Glyceraldehyde-3-phosphate (3C), producing Erythrose-4-phosphate (4C) and Fructose-6-phosphate (6C).
c) Second Transketolase Reaction
- Enzyme: Transketolase (again, requires TPP)
- Reaction: Transfers 2 carbons from another Xylulose-5-phosphate (5C) to Erythrose-4-phosphate (4C), producing another Glyceraldehyde-3-phosphate (3C) and another Fructose-6-phosphate (6C).
Overall Summary of the Non-Oxidative Phase
If 3 molecules of glucose-6-phosphate enter the oxidative phase, they produce 3 molecules of ribulose-5-phosphate and 6 NADPH. These 3 molecules of ribulose-5-phosphate are then processed through the non-oxidative phase:
These glycolytic intermediates can then enter glycolysis, be used for gluconeogenesis, or be recycled to continue the PPP.
Flexibility of the PPP
The reversibility of the non-oxidative phase is key, allowing the pathway to operate in different modes:
- If the cell needs more NADPH than ribose-5-phosphate: The oxidative phase is active, and pentose phosphates are recycled back to glucose-6-phosphate to maintain the flow.
- If the cell needs more ribose-5-phosphate than NADPH: The oxidative phase can be bypassed, and glycolytic intermediates can enter the non-oxidative phase in reverse to produce ribose-5-phosphate.
- If the cell needs both NADPH and ATP: The oxidative phase produces NADPH, and the non-oxidative phase converts pentose phosphates into F6P and G3P, which then enter glycolysis for ATP production.
Primary Tissues/Cells of Activity
The activity of the PPP varies significantly among different tissues, directly reflecting their metabolic demands for its key products: NADPH and ribose-5-phosphate.
Tissues with High Demand for NADPH:
Liver (Hepatocytes)
The liver is a central metabolic hub with a high demand for NADPH for:
- Fatty acid synthesis
- Cholesterol and steroid synthesis
- Drug detoxification (cytochrome P450 system)
Adipose Tissue (Adipocytes)
Adipocytes are specialized for fat storage and have a very high demand for NADPH to support the massive amount of fatty acid synthesis that occurs here.
Red Blood Cells (Erythrocytes)
RBCs lack mitochondria and are constantly exposed to oxidative stress. The PPP is their only source of NADPH for antioxidant defense, used to maintain reduced glutathione (GSH) and protect the cell.
Steroidogenic Tissues
Tissues like the adrenal cortex, testes, and ovaries are primary sites of steroid hormone synthesis and have a high demand for NADPH for these hydroxylation reactions.
Mammary Gland (Lactating)
During lactation, the mammary gland synthesizes large amounts of fatty acids for milk production, requiring a high supply of NADPH.
Tissues with High Demand for Ribose-5-Phosphate:
Rapidly Dividing Cells
Tissues like bone marrow, skin, intestinal mucosa, and tumors are continuously proliferating and require constant DNA and RNA synthesis. They have a high demand for ribose-5-phosphate for nucleotide synthesis.
The non-oxidative phase can be reversed in these cells to primarily produce ribose-5-phosphate from glycolytic intermediates.
Regulation
The regulation of the Pentose Phosphate Pathway primarily occurs at its committed and rate-limiting step, catalyzed by Glucose-6-Phosphate Dehydrogenase (G6PD). The non-oxidative phase is primarily driven by substrate availability.
1. Regulation of Glucose-6-Phosphate Dehydrogenase (G6PD)
G6PD is the most important regulatory enzyme of the PPP. Its activity is controlled by:
a) Substrate Availability (Glucose-6-Phosphate)
Higher levels of G6P generally lead to increased G6PD activity.
b) Product Inhibition by NADPH (The Primary Regulator)
- NADPH is a potent competitive inhibitor of G6PD. This is the most crucial regulatory mechanism.
- When the cellular concentration of NADPH is high, it binds to G6PD and inhibits its activity, reducing further NADPH production.
- Conversely, when NADPH is low (and NADP⁺ is high), inhibition is relieved, and G6PD activity increases.
- Therefore, the ratio of NADPH/NADP⁺ is the primary determinant of the flux through the oxidative phase.
c) Transcriptional Regulation (Gene Expression)
The synthesis of G6PD can be regulated at the gene expression level. For example, a high-carbohydrate diet and insulin can lead to an increase in the synthesis of G6PD, increasing the capacity to produce NADPH for fatty acid synthesis.
2. Regulation of the Non-Oxidative Phase
The reversible reactions are primarily regulated by the availability of substrates and the cell's demand for products.
- If the cell needs ribose-5-phosphate, the equilibrium shifts towards its production.
- If the cell needs to recycle carbons back into glycolysis, the equilibrium shifts towards F6P and G3P.
3. Interplay with Glycolysis
The PPP and glycolysis compete for the common substrate, glucose-6-phosphate.
- High demand for NADPH and/or ribose-5-phosphate directs G6P into the PPP.
- High demand for ATP favors glycolysis.
Physiological Roles of NADPH
NADPH, produced almost exclusively by the PPP, plays essential roles in maintaining cellular homeostasis and facilitating various metabolic processes.
Reductive Biosynthesis
NADPH provides the electrons (reducing power) necessary for many synthetic (anabolic) reactions. Key examples include:
- Fatty Acid Synthesis: A major consumer of NADPH in the liver, adipose tissue, and lactating mammary gland.
- Cholesterol and Steroid Hormone Synthesis: Involves several NADPH-dependent reduction steps in the liver, adrenal cortex, and gonads.
- Deoxyribonucleotide Synthesis: The conversion of ribonucleotides to deoxyribonucleotides for DNA synthesis ultimately relies on NADPH.
Antioxidant Defense
NADPH is crucial for protecting cells from damage by Reactive Oxygen Species (ROS). It maintains the cellular defense system through its role in the glutathione system.
- Glutathione Reductase: This enzyme uses NADPH to reduce oxidized glutathione (GSSG) back to its protective, reduced form (GSH).
GSSG + NADPH + H⁺ → 2 GSH + NADP⁺ - Glutathione Peroxidase: Reduced glutathione (GSH) is then used to detoxify hydrogen peroxide (H₂O₂) by converting it into water.
2 GSH + H₂O₂ → GSSG + 2 H₂O
Phagocytosis (Respiratory Burst)
In phagocytic immune cells (e.g., neutrophils), NADPH plays a critical role in the "respiratory burst."
NADPH Oxidase: This enzyme uses NADPH to produce superoxide radicals (O₂•⁻), which are then converted into other potent oxidants (like hydrogen peroxide) to kill engulfed bacteria and pathogens.
O₂ + NADPH → O₂•⁻ + NADP⁺ + H⁺Significance of the Hexose Monophosphate (HMP) Shunt / Pentose Phosphate Pathway (PPP)
The HMP Shunt holds paramount significance due to its unique role in generating two crucial products: pentoses and NADPH. Unlike glycolysis, its value lies in providing essential building blocks and reducing power for various anabolic and protective processes.
I. Importance of Pentoses
The HMP shunt converts hexoses into pentose sugars, with ribose-5-phosphate being the most important. These are indispensable for:
- Nucleic Acid Synthesis: Ribose-5-phosphate is a direct precursor for the ribose in RNA and, after reduction, the deoxyribose in DNA.
- Nucleotide Coenzyme Synthesis: Ribose is necessary for synthesizing vital coenzymes such as ATP, NAD⁺, FAD, and Coenzyme A.
II. Importance of NADPH
NADPH is a versatile reducing agent, distinct from NADH, and serves as a critical source of electrons for a wide array of anabolic and protective cellular functions.
1. Reductive Biosynthesis
NADPH provides reducing power for building complex molecules like fatty acids, cholesterol, steroid hormones, and amino acids.
2. Antioxidant Defense
NADPH is critical for regenerating reduced glutathione (GSH), which is used by glutathione peroxidase to neutralize harmful free radicals and peroxides, protecting cells from oxidative damage.
3. Erythrocyte Membrane Integrity
In red blood cells, the concerted action of NADPH and the glutathione system is vital for preserving the integrity of the cell membrane, protecting it from oxidative damage and preventing premature lysis (hemolytic anemia).
4. Prevention of Met-Hemoglobinemia
NADPH-dependent reductase systems are essential for keeping the iron within hemoglobin in its reduced (ferrous, Fe²⁺) state. This prevents the formation of met-hemoglobin (Fe³⁺), which cannot carry oxygen.
5. Detoxification of Drugs
The liver's microsomal cytochrome P450 monooxygenase system depends on NADPH to detoxify drugs and foreign substances by increasing their solubility for excretion.
6. Preservation of Lens Transparency
The eye's lens has a high concentration of NADPH, which is vital for protecting lens proteins from oxidative damage, thereby guarding against conditions like cataracts.
7. Macrophage Bactericidal Activity
In phagocytic cells, NADPH oxidase uses NADPH to generate large quantities of superoxide radicals in a process called the "respiratory burst." These reactive oxygen species are potent antimicrobial agents used to kill ingested bacteria.
Biochemistry: Pentose Phosphate Pathway
Test your knowledge with these 35 questions.
PPP Exam
Question 1/35
Exam Complete!
Here are your results, .
Your Score
33/35
94%