Bioenergetics &: Metabolism
Bioenergetics
Bioenergetics is the specialized field that studies how living organisms acquire, transform, and utilize energy. It's essentially the application of the principles of thermodynamics – the study of energy and its effects on matter – to biological processes.
Concepts of Energy
Energy: At its core, energy is the capacity to do work. In biological systems, "work" can encompass a vast array of activities: muscle contraction, nerve impulse transmission, synthesizing complex molecules, maintaining body temperature, and transporting substances across cell membranes.
Forms of Energy:
- Kinetic Energy: The energy of motion. Examples include heat energy, light energy, and mechanical energy (e.g., a moving muscle).
- Potential Energy: Stored energy, or the energy of position. This is the energy that could do work. In biology, the most crucial form is chemical energy, stored within the bonds of molecules like ATP, glucose, and fats.
Thermodynamics and Chemical Reactions
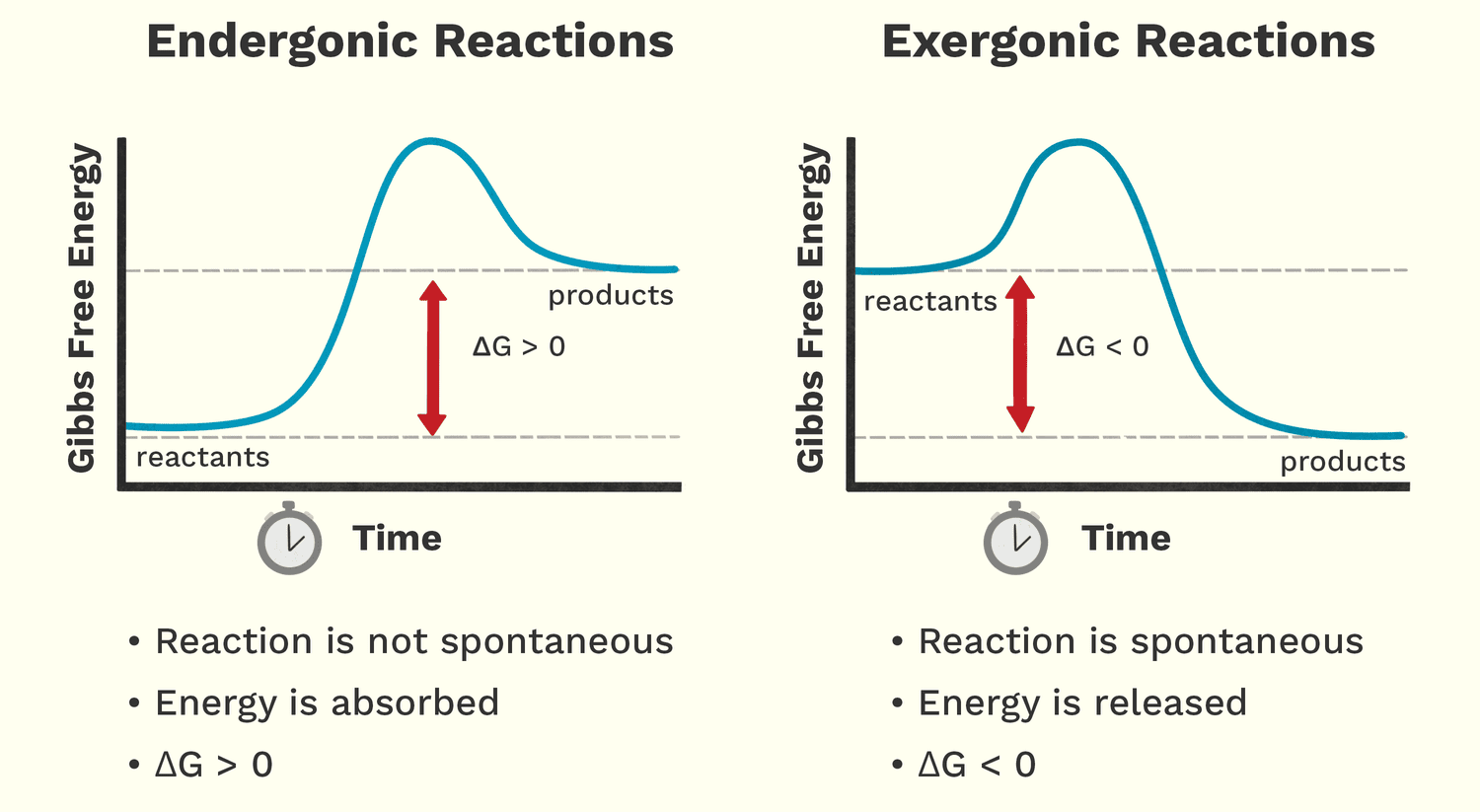
Thermodynamics provides the framework for understanding energy changes during chemical reactions.
Exergonic vs. Endergonic Reactions:
- Exergonic Reactions (Energy-Releasing): These reactions release energy into the surroundings. They are spontaneous.
- Endergonic Reactions (Energy-Consuming/Requiring): These reactions require an input of energy to proceed. They are non-spontaneous.
Free Energy (ΔG): The "Usable" Energy
- Free Energy (G): Represents the portion of a system's energy that is available to do work.
-
Change in Free Energy (
ΔG): The difference in free energy between the products and reactants.- A negative
ΔG: The reaction is exergonic and spontaneous. - A positive
ΔG: The reaction is endergonic and non-spontaneous. ΔG = 0: The reaction is at equilibrium.
- A negative
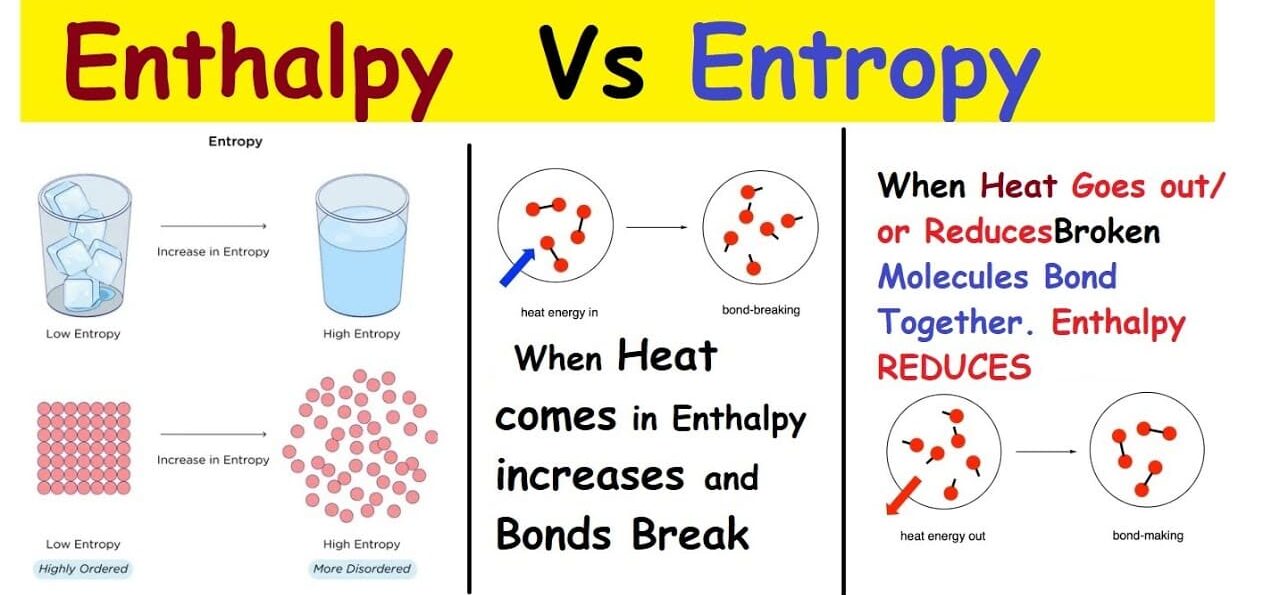
Enthalpy and Entropy:
The change in free energy (
ΔG) is determined by two other thermodynamic quantities: Enthalpy and Entropy. The relationship is expressed by the Gibbs Free Energy Equation:
Where:
ΔG= Change in Free EnergyΔH= Change in EnthalpyT= Absolute Temperature (in Kelvin)ΔS= Change in Entropy
-
Enthalpy (
ΔH): Represents the change in heat content.- Negative
ΔH(Exothermic): Heat is released. - Positive
ΔH(Endothermic): Heat is absorbed.
- Negative
-
Entropy (
ΔS): Represents the change in randomness or disorder. The universe tends towards maximum entropy (Second Law of Thermodynamics).- Positive
ΔS: The system becomes more disordered. - Negative
ΔS: The system becomes more ordered.
- Positive
In biological systems, reactions often lead to a temporary decrease in entropy locally (e.g., building a complex protein). However, this is always accompanied by a greater increase in disorder in the surroundings, maintaining the Second Law of Thermodynamics overall.
Standard Free Energy (ΔG°) and Biological Standard Free Energy (ΔG°')
-
Standard Free Energy (
ΔG°): The change in free energy under standard conditions (1 M concentration, 25° C, 1 atm pressure). -
Biological Standard Free Energy (
ΔG°'): In biology, a modified standard condition is used to better reflect physiological conditions:- pH 7.0 (neutral).
- All other conditions remain the same as
ΔG°.
The actual free energy change (ΔG) in a living cell will depend on the actual concentrations of reactants and products. However, ΔG°' is a useful reference point.
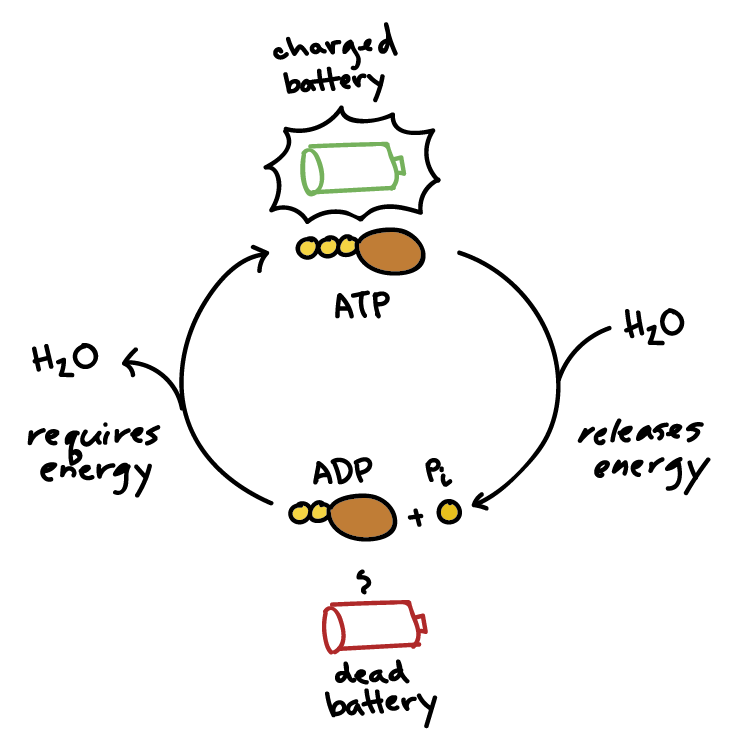
Biological Energy Transformations: Coupled Reactions
Living organisms power endergonic reactions through coupled reactions, where the liberation of energy from an exergonic reaction is used to drive an endergonic one.
ATP Hydrolysis: A Classical Example of an Exergonic Reaction:
The hydrolysis of Adenosine Triphosphate (ATP) to Adenosine Diphosphate (ADP) and inorganic phosphate (Pi) is a highly exergonic reaction, releasing a significant amount of free energy (approx. -7.3 kcal/mol or -30.5 kJ/mol under standard biological conditions, ΔG°'). This released energy is then used to fuel various endergonic processes in the cell.
Additive Nature of Free Energy Changes in Pathways
Biochemical pathways consist of a series of sequential reactions. The overall free energy change (ΔG) for an entire pathway is the sum of the ΔG values of all individual reactions.
Crucially, even if some individual reactions are endergonic (positive ΔG), the pathway can still proceed as long as the sum of all ΔG values for the entire pathway is negative. This is achieved by coupling endergonic steps with highly exergonic steps, effectively "pulling" the pathway forward.
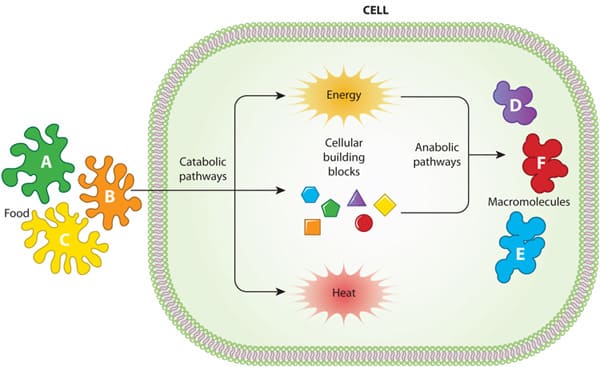
Cellular Metabolic Reactions
Metabolism refers to all the chemical reactions that occur in living organisms to maintain life. These processes allow organisms to grow, reproduce, maintain their structures, and respond to their environments. Cellular metabolism is a highly organized and interconnected network of reactions that takes place within the cell.
Types of Metabolism
There are two main types of metabolic reactions:
-
Catabolism (Breakdown): The process of breaking down complex molecules into simpler ones, usually releasing energy in the process. This energy is then captured and stored in molecules like ATP (adenosine triphosphate).
- Analogy: Demolition – taking apart a complex building to get raw materials and energy.
- Example: The breakdown of glucose into carbon dioxide and water to produce ATP.
-
Anabolism (Build-up/Synthesis): The process of building complex molecules from simpler ones, which typically requires an input of energy (often supplied by ATP).
- Analogy: Construction – using raw materials and energy to build a complex structure.
- Example: The synthesis of proteins from amino acids, or DNA from nucleotides.
These two processes are linked: the energy released during catabolism fuels anabolism, creating a continuous cycle of energy transformation and matter recycling within the cell.
Characteristics of Cellular Metabolic Reactions
- Enzyme-Catalyzed: Almost all metabolic reactions are catalyzed by specific enzymes. Enzymes allow reactions to occur quickly and efficiently at physiological temperatures and pH.
-
Highly Regulated: Metabolic pathways are tightly controlled to ensure that cells only produce what they need, when they need it. Regulation occurs at various levels:
- Enzyme activity: Allosteric regulation, feedback inhibition, covalent modification (e.g., phosphorylation).
- Enzyme synthesis: Gene expression can be turned on or off.
- Substrate availability: The presence or absence of reactants can dictate reaction rates.
-
Occur in Pathways: Metabolic reactions are rarely isolated events. Instead, they occur in a series of sequential, interconnected steps called metabolic pathways. The product of one reaction often serves as the substrate for the next.
- Linear pathways:
A → B → C → D - Branched pathways:
A → B → CandA → B → D - Cyclic pathways:
A → B → C → A(e.g., Krebs cycle)
- Linear pathways:
- Energy Transformations: A central theme of metabolism is energy transformation. Cells capture energy from their environment (from sunlight or food) and convert it into a usable form, primarily ATP.
-
Location-Specific: Many metabolic pathways are compartmentalized within specific organelles of the cell. This allows for efficient regulation and prevents conflicting reactions from occurring simultaneously.
- Cytosol: Glycolysis, pentose phosphate pathway.
- Mitochondria: Krebs cycle, oxidative phosphorylation, fatty acid oxidation.
- Endoplasmic Reticulum: Lipid synthesis, protein folding.
- Lysosomes: Degradation of macromolecules.
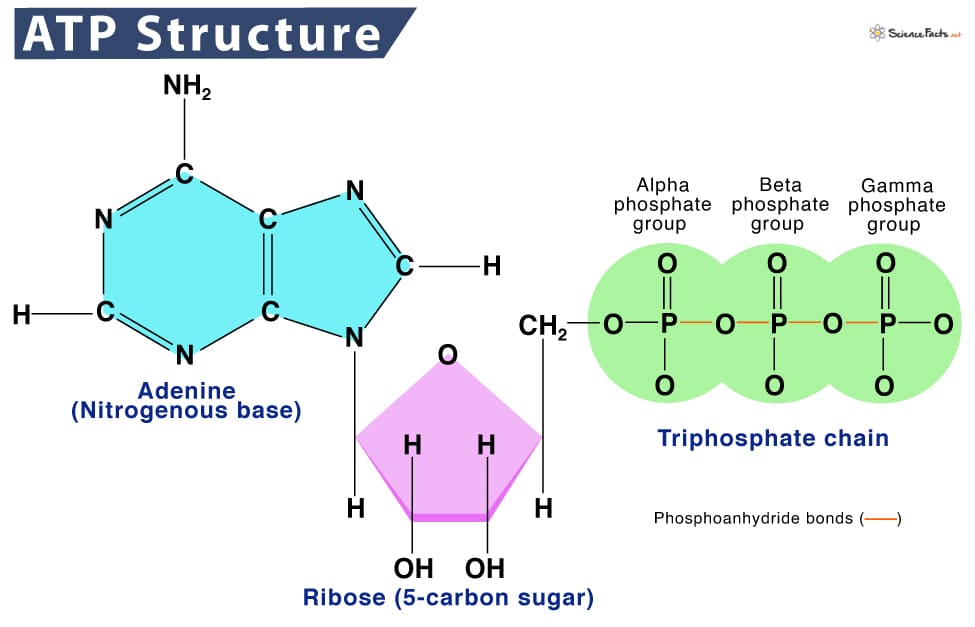
The Central Role of ATP (Adenosine Triphosphate)
ATP is often called the "energy currency" of the cell. It's a nucleotide consisting of adenine, a ribose sugar, and three phosphate groups. The energy stored in ATP is primarily in the high-energy phosphate bonds.
-
ATP Hydrolysis: When the terminal phosphate bond of ATP is broken (hydrolyzed) to form ADP (adenosine diphosphate) and inorganic phosphate (Pi), a significant amount of free energy is released (exergonic reaction).
ATP + H₂O → ADP + Pi + Energy -
ATP Synthesis: The reverse reaction, the synthesis of ATP from ADP and Pi, requires energy input (endergonic reaction) and is typically coupled to energy-releasing catabolic processes.
ADP + Pi + Energy → ATP
ATP provides the energy for a wide range of cellular activities, including:
- Muscle contraction
- Active transport across membranes
- Synthesis of macromolecules (anabolism)
- Nerve impulse transmission
- Maintaining body temperature
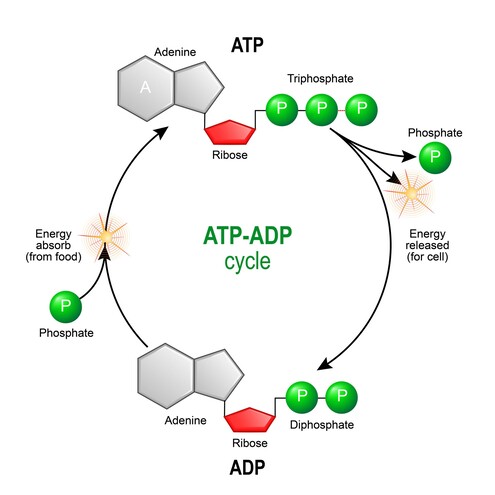
How ATP is Made: The Two Main Strategies
Cells primarily use two distinct strategies to "recharge" ADP into ATP: Substrate-Level Phosphorylation and Oxidative Phosphorylation.
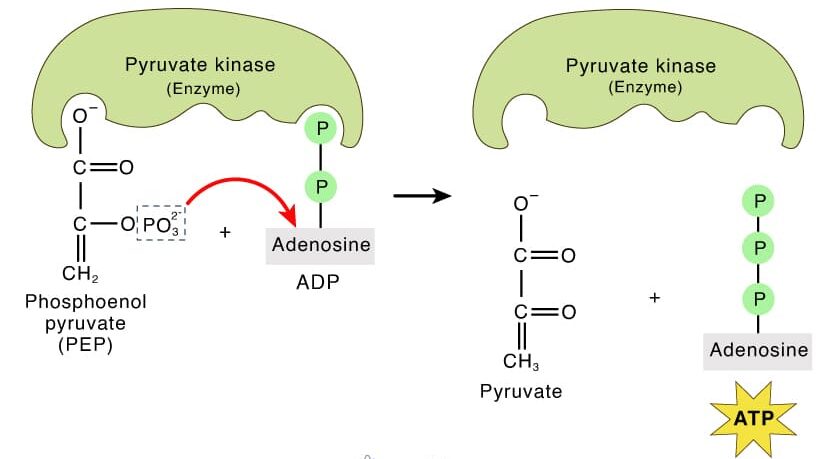
A. Substrate-Level Phosphorylation: The "Direct Hand-Off" Method:
- The Concept: Imagine a relay race where one runner directly hands the baton (a phosphate group) to the next runner (ADP) who then crosses the finish line (becomes ATP).
- Mechanism: It's a metabolic reaction where an enzyme directly transfers a phosphate group from a high-energy donor compound (the "substrate") to an ADP molecule, forming ATP. No complex electron transfers or oxygen are directly involved.
-
Key Features:
- Direct Transfer: The phosphate comes straight from another molecule.
- Quicker Source: It's a relatively fast way to produce ATP.
- Lower Yield: It only generates a small amount of ATP.
- Exergonic Reaction: The breaking of the high-energy bond in the substrate molecule releases enough energy to power the formation of ATP.
-
Where it Happens:
- Cytoplasm (during Glycolysis): Examples include the conversion of 1,3-bisphosphoglycerate to 3-phosphoglycerate and phosphoenolpyruvate to pyruvate.
- Mitochondria (during the Krebs Cycle): The conversion of succinyl-CoA to succinate yields GTP, which is quickly converted to ATP.
- A "substrate" molecule with a phosphate group attached passes that phosphate directly to an ADP molecule, creating ATP. The enzyme simply facilitates this direct transfer.
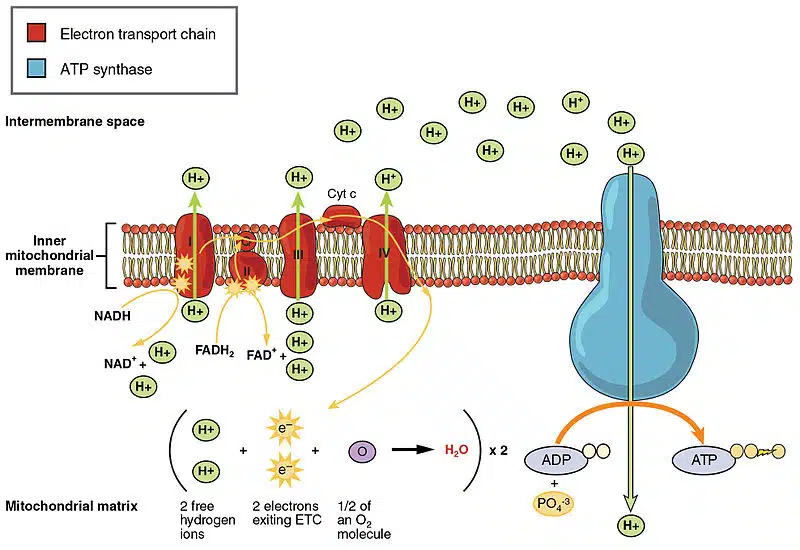
B. Oxidative Phosphorylation: The "Conveyor Belt" Energy Factory:
- The Concept: This is the cell's main power plant, producing the vast majority of ATP. It uses a sophisticated system of electron transport and a "proton pump" to generate energy.
- Mechanism: A complex series of reactions that uses the energy from electrons (stripped from food molecules) to create a proton gradient, which then drives ATP synthesis.
-
Key Features:
- Major Source of ATP: Generates most of the ATP in aerobic organisms.
- Aerobic Respiration Hallmark: It absolutely requires oxygen.
- Highly Conserved: Found in nearly all complex life forms.
- Where it Happens: Exclusively within the mitochondria, specifically on the inner mitochondrial membrane.
How it Works (The "Conveyor Belt" Analogy):
-
Electron Delivery (NADH & FADH₂): Imagine tiny delivery trucks,
NADHandFADH₂, loaded with high-energy electrons from the breakdown of food. They arrive at the inner mitochondrial membrane. - The Electron Transport Chain (ETC): These trucks unload their electrons onto a series of protein complexes called the ETC. Think of it as a conveyor belt. As electrons "fall" down this chain, they release small bursts of energy.
-
Proton Pumping & Electrochemical Gradient: The energy released is used by some complexes to act as proton pumps, forcing protons (H⁺ ions) from the mitochondrial matrix into the intermembrane space. This creates a highly concentrated "pool" of protons, a difference in charge and concentration known as an electrochemical gradient.
Analogy: This is like pumping water uphill to create a reservoir behind a dam. The stored water (protons) has potential energy. -
ATP Synthase (The "Turbine"): The accumulated protons flow back across the membrane through a specific channel: the enzyme ATP synthase. This enzyme acts like a tiny turbine. As protons flow through it, the enzyme spins, driving the reaction of combining ADP and Pi to synthesize a large amount of ATP.
Analogy: The water in the reservoir flows down through a turbine (ATP synthase) to generate electricity (ATP). - Oxygen's Role: At the very end of the ETC, the electrons combine with protons and oxygen to form water. Oxygen is the final electron acceptor. Without it, the ETC would back up, and ATP production would halt.
What Oxidative Phosphorylation Produces:
- Lots of ATP: This is its primary and most vital product.
-
Reactive Oxygen Species (ROS): As a side effect, the ETC can sometimes prematurely pass electrons to oxygen, leading to the formation of highly reactive molecules like superoxide and hydrogen peroxide.
Analogy: Think of a factory with some exhaust fumes. While efficient, there are inevitable byproducts. - Impact of ROS: While cells have defenses against ROS, an excess can be damaging. ROS can harm DNA, proteins, and lipids, contributing to aging and various diseases.
Biological Oxidation, the Electron Transport Chain, and Oxidative Phosphorylation
Imagine your body as a high-performance engine, and food as its fuel. Just as an engine burns fuel to produce mechanical energy, your cells "burn" food molecules to produce the chemical energy currency: ATP. This isn't a quick, explosive burn, however. It's a highly controlled process called Biological Oxidation, culminating in the Electron Transport Chain (ETC) and Oxidative Phosphorylation – the cellular equivalent of a meticulously managed power plant.
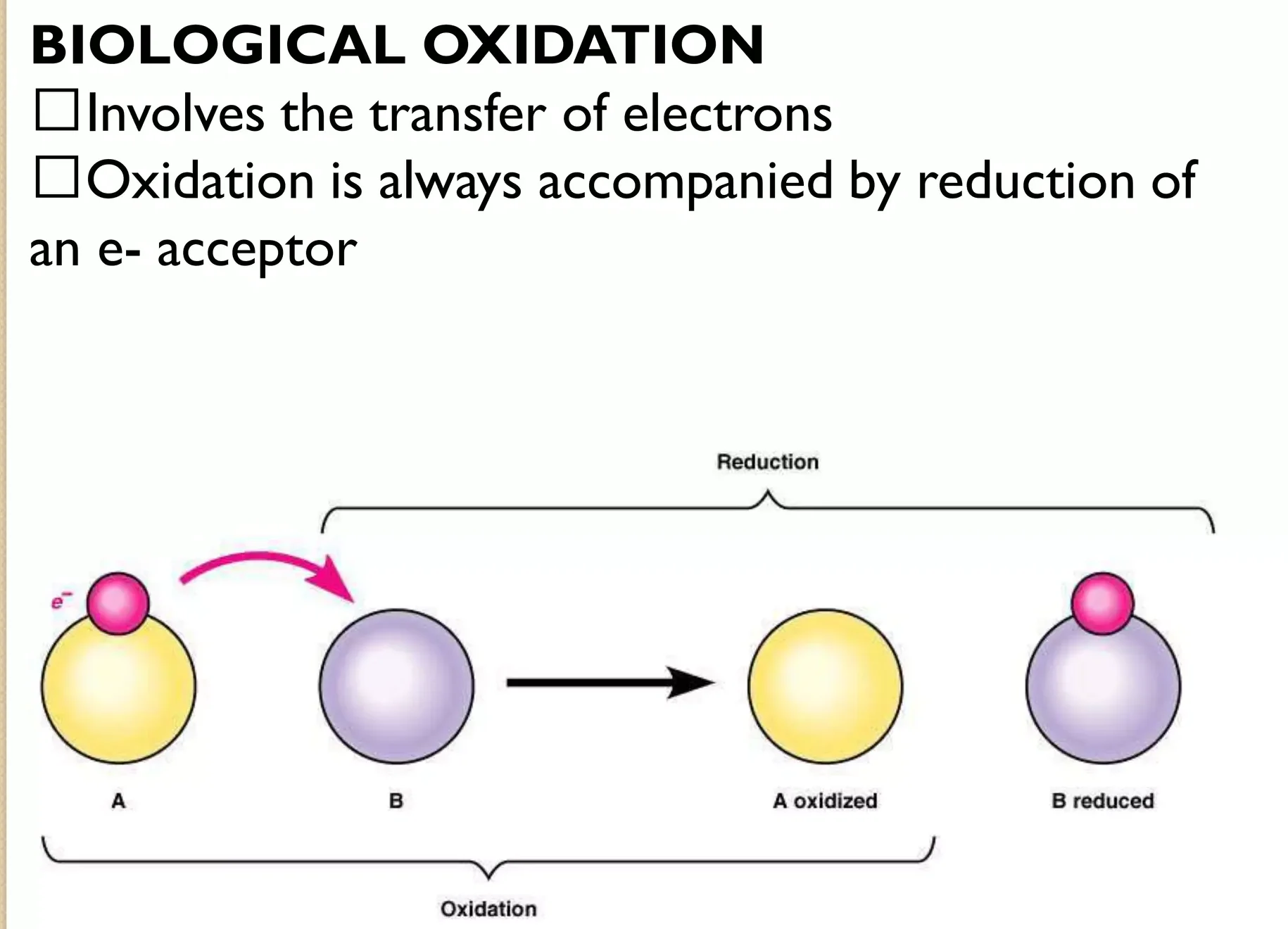
I. Biological Oxidation:
Before we get to the power plant, we need to understand the concept of "burning" food in biology. This isn't fire; it's a series of chemical reactions where molecules lose electrons.
Oxidation vs. Reduction – The Dance of Electrons:
- Oxidation: In chemistry, oxidation means a molecule loses electrons.
- Reduction: Conversely, reduction means a molecule gains electrons.
- They're Inseparable: Oxidation and reduction always happen together. If one molecule is oxidized, another must be reduced. These are called redox reactions.
- In Living Systems: Often, biological oxidation involves the removal of hydrogen atoms (a proton and an electron).
Analogy: Imagine a person (a molecule) carrying a heavy backpack (hydrogen atoms/electrons). When they "oxidize," they pass the backpack to someone else. They become lighter (oxidized), and the person who receives it becomes heavier (reduced).
The Energy Principle: Your food molecules (like glucose) are rich in electrons. By gradually removing these electrons (oxidizing the food), cells can harvest the energy stored within them.
Example: The transformation of ferrous iron (Fe²⁺) to ferric iron (Fe³⁺):
Here, Fe²⁺ is oxidized. This electron then moves on to reduce another molecule.
The Electron Shuttles: Cells use special "electron taxis" or "shuttles" like NADH (from NAD⁺) and FMNH₂ (from FMN). These molecules pick up electrons (become reduced) from the breakdown of food and deliver them to the Electron Transport Chain.
II. Overview of the Electron Transport Chain:
The ETC is where energy-rich electrons converge to generate ATP.
- Fueling the System: Carbohydrates, fats, and amino acids are first broken down.
- Making the "Electron Taxis": During this breakdown, electrons are extracted and loaded onto
NADHandFADH₂. Think of these as fully charged battery packs. - The Power Plant (ETC): These charged taxis deliver their electrons to the ETC, a series of protein complexes that act like a miniature hydroelectric dam.
- The Flow of Energy: As electrons "fall" through this chain, their energy is gradually released.
- Oxygen: The Final Acceptor: At the end of the line, the spent electrons are accepted by oxygen (O₂), combining with protons (H⁺) to form water (H₂O). This highlights why we breathe oxygen.
- ATP Generation: The energy released from this electron flow is then used to synthesize large amounts of ATP.
- Waste Products: The final "exhaust" from burning our food is carbon dioxide (CO₂) and water (H₂O).
III. Metabolic Breakdown of Energy-Yielding Molecules: Collecting the Good Stuff
How different food sources contribute to the ETC.
- Food as Raw Material: Sugar, fat, or protein are broken down through various metabolic pathways (glycolysis, Krebs cycle, beta-oxidation).
- Harvesting Electrons: The key output is not directly ATP, but rather our "energy-rich reduced coenzymes" – predominantly
NADHandFADH₂. These molecules are the direct carriers of the energy that will feed the ETC. - Heat Generation: Not all energy is captured perfectly. Some is always dissipated as heat. This "excess energy generates heat," which helps maintain our body temperature.
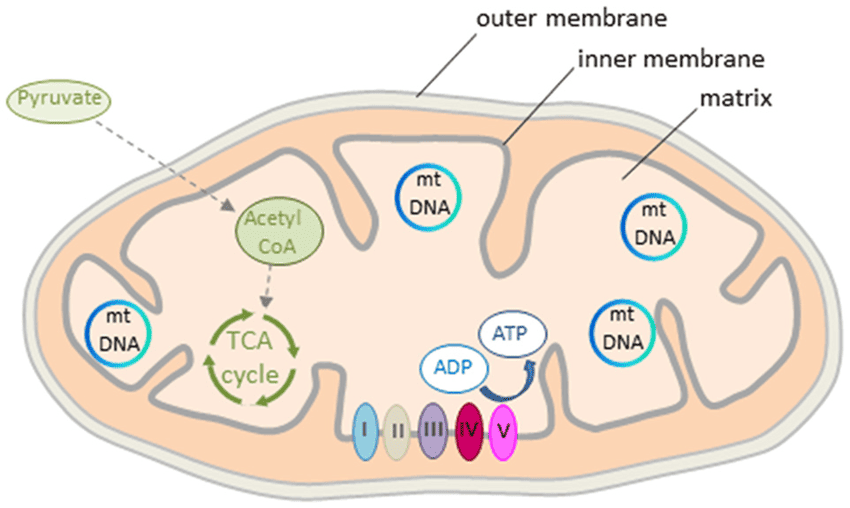
IV. The Mitochondria:
The entire process of the ETC and oxidative phosphorylation is confined to a specialized organelle: the mitochondrion.
- The "Power House" Design: Mitochondria are the "powerhouses of the cell" because they generate most of the cell's ATP.
- Mitochondrial Organization (Like a Factory Layout):
- Outer Membrane: The factory's outer wall. It's relatively porous.
- Intermembrane Space: The corridor between the outer and inner walls. This space is for building up a high concentration of protons.
- Inner Mitochondrial Membrane: The most important part for ATP production. It's highly selective and impermeable to most ions (like H⁺, K⁺, Na⁺). This impermeability is vital for maintaining the proton gradient.
- Cristae: Increasing Efficiency: The inner membrane is intricately folded into shelf-like structures called cristae.
- Analogy: Imagine building shelves in a small factory to maximize space. The cristae increase the surface area of the inner membrane, allowing more room for the ETC complexes and ATP synthase enzymes.
- Mitochondrial Matrix: The factory floor, the innermost compartment. This is a busy place, rich in enzymes that carry out:
- The citric acid cycle (Krebs cycle)
- Beta-oxidation of fatty acids
- Oxidation of amino acids
Essentially, the matrix is where much of the initial "fuel processing" happens to prepare electrons for the ETC.
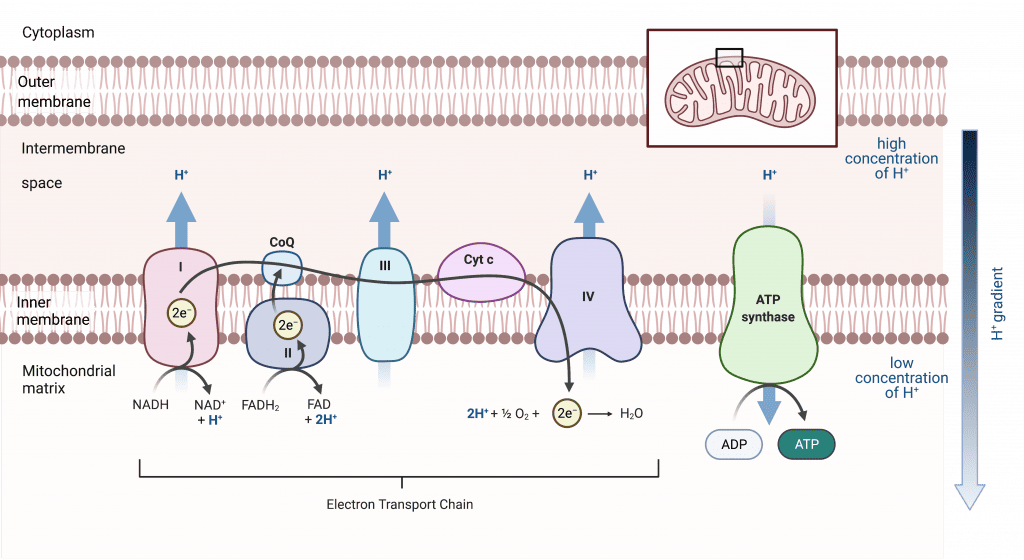
V. The Electron Transport Chain (ETC):
Located entirely within the inner mitochondrial membrane.
The ETC is a precisely arranged series of protein complexes and mobile electron carriers that work together like a bucket brigade or an assembly line.
- The Goal: To take electrons from NADH and FADH₂, pass them down a chain, and use the energy released to make ATP.
- The Components (The "Workers" on the Assembly Line):
- Four Major Protein Complexes (I, II, III, IV): Large, multi-subunit proteins embedded in the inner membrane.
- Complex V (ATP Synthase): The final machine that synthesizes ATP.
- Two Mobile Carriers (Coenzyme Q and Cytochrome c): Small delivery carts ferrying electrons between the larger complexes.
- The Flow:
- Electron Acceptance: Each complex in the chain accepts electrons from the preceding component.
- Electron Donation: It then donates those electrons to the next component.
- The "Electron Affinity Gradient": The complexes are arranged in a specific order of increasing electron affinity, ensuring a downhill flow of electrons.
- Oxygen: The Ultimate Magnet: Electrons keep moving down this energy gradient until they reach the end, where they combine with oxygen (our final electron acceptor) to form water.
- Energy Harvest: The gradual release of energy as electrons move down this chain is harnessed to pump protons and ultimately make ATP.
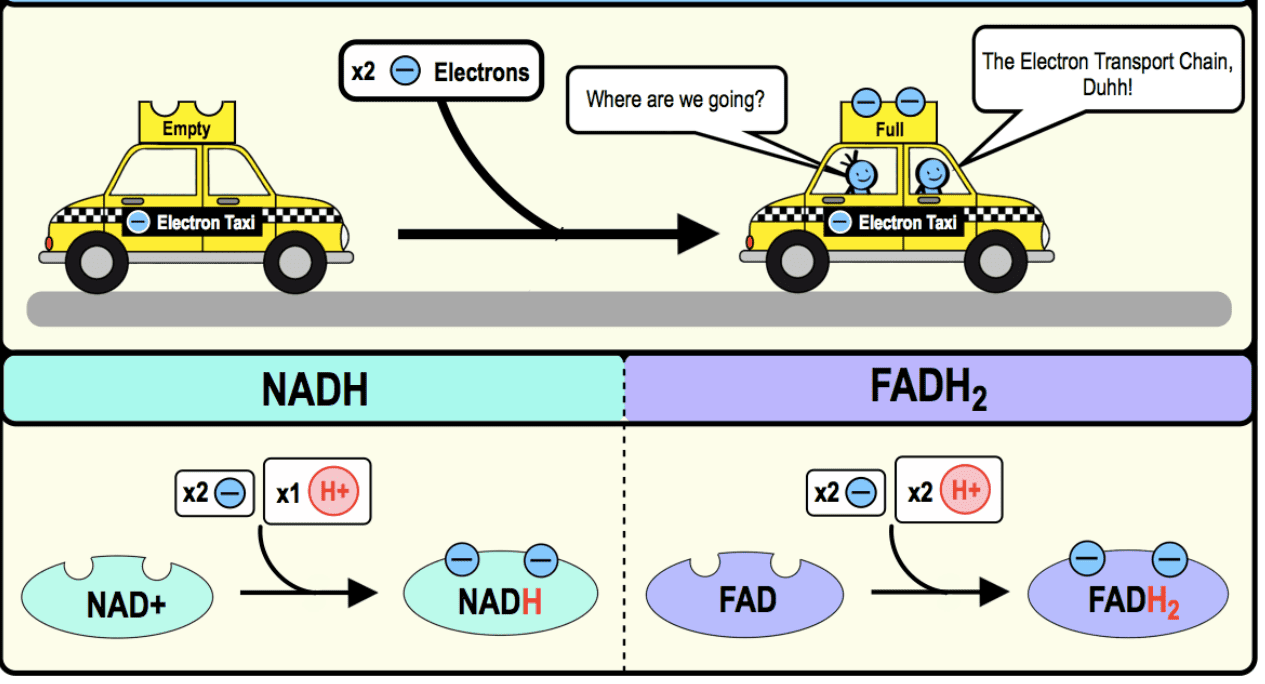
VI. The Electron Carriers: NADH, FADH₂, and More
These are the molecules that bring and pass electrons through the ETC.
-
NADH and FADH₂: The Primary Electron Taxis:
-
Nicotinamide Adenine Dinucleotide (NAD⁺/NADH):
- Derived from niacin (Vitamin B3).
- NADH is the reduced form (carrying 2 electrons) and is primarily involved in the ETC.
-
Flavin Adenine Dinucleotide (FAD/FADH₂):
- Derived from riboflavin (Vitamin B2).
- FADH₂ is the reduced form and also carries 2 electrons to the ETC.
- NADPH: Related to NADH but used for building molecules (anabolic reactions), not typically for ETC.
-
Nicotinamide Adenine Dinucleotide (NAD⁺/NADH):
-
Complex I: NADH Dehydrogenase – The First Port of Entry
- This is where NADH drops off its electrons.
- It contains the enzyme NADH dehydrogenase, a flavoprotein (contains FMN) with iron-sulfur clusters.
- The Reaction:
NADH + H⁺ + FMN → NAD⁺ + FMNH₂. Electrons are passed to Coenzyme Q (CoQ). - Crucial Action: Complex I uses the energy from these electrons to pump protons (H⁺) from the matrix into the intermembrane space.
-
Complex II: Succinate Dehydrogenase – A Unique Entry Point
- This is where FADH₂ delivers its electrons.
- It is called Succinate dehydrogenase, unique because it's both an enzyme in the Krebs cycle and a component of the ETC.
- The Reaction:
Succinate + FAD → Fumarate + FADH₂. FADH₂ is formed directly within Complex II. - Electrons are passed to Coenzyme Q (CoQ).
- Important Difference: Unlike Complex I, Complex II does NOT pump protons. This is why FADH₂ contributes less to ATP production than NADH.
-
Coenzyme Q (CoQ) / Ubiquinone – The Mobile Shuttle
- CoQ is a small, lipid-soluble molecule that acts like a ferry boat, moving within the inner mitochondrial membrane.
- It collects electrons from both Complex I and Complex II.
- It then delivers these electrons to Complex III.
-
Cytochromes – The Iron Carriers
- Cytochromes are proteins that contain an iron-containing heme group.
- The iron atom can switch between Ferric (Fe³⁺, oxidized) and Ferrous (Fe²⁺, reduced) states.
- This reversible change (
Fe³⁺ ↔ Fe²⁺) allows them to efficiently pick up and release electrons one at a time.
-
Complex III and IV: The Final Steps
- Complex III (Cytochrome bc₁ complex):
- Receives electrons from CoQ.
- Passes electrons to Cytochrome c (another mobile carrier).
- Crucially, Complex III also pumps protons.
- Complex IV (Cytochrome c oxidase):
- Receives electrons from Cytochrome c.
- This is the terminal complex. Here, electrons are finally passed to oxygen (O₂), which combines with protons to form water (H₂O).
- Crucially, Complex IV also pumps protons.
- Complex III (Cytochrome bc₁ complex):
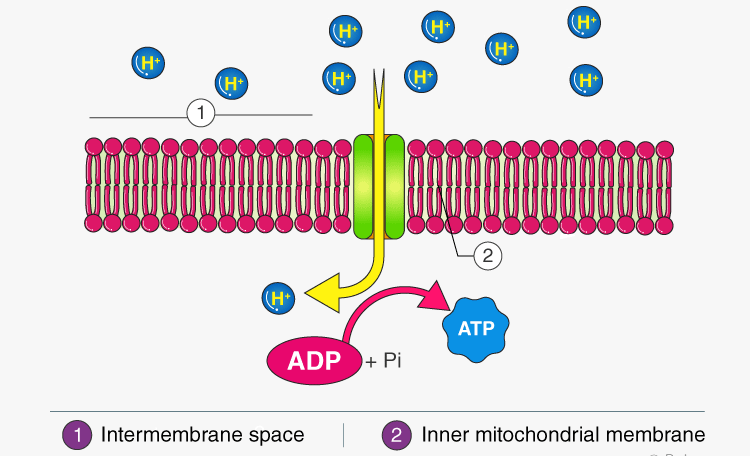
VII. Oxidative Phosphorylation: Turning the Proton Flow into ATP
The "oxidative" part (electron transport) is now coupled to the "phosphorylation" part (making ATP).
- The Link: The transport of electrons through Complexes I, III, and IV releases free energy, which is used to pump protons.
-
The Proton Gradient – A Stored Battery:
- As electrons move, Complexes I, III, and IV act as proton pumps, moving H⁺ ions from the matrix to the intermembrane space.
- Analogy: This is like using energy to pump water uphill into a reservoir. You're creating a high concentration of water (protons) on one side with potential energy. This difference in proton concentration is the electrochemical proton gradient.
- The Chemiosmotic Hypothesis: Proposed by Peter Mitchell, this explains how the proton gradient drives ATP synthesis. "Chemi" refers to the chemical gradient, and "osmotic" to movement across a membrane.
-
ATP Synthase (Complex V) – The Turbine:
- The inner membrane is impermeable to protons. The only way for protons to flow back down their gradient is through ATP synthase.
- Analogy: ATP synthase is like the turbine at the base of our dam. As the protons (water) flow back down, the turbine spins.
- ATP Synthesis: The spinning of ATP synthase uses this mechanical energy to catalyze the formation of ATP from ADP and Pi.
-
Sites of Oxidative Phosphorylation (Proton Pumping Sites):
- There are three main "power strokes" or sites where enough energy is released to pump protons:
- At Complex I, when NADH donates electrons.
- At Complex III, as electrons move from CoQ to cytochrome c.
- At Complex IV, as electrons finally reach oxygen.
The Mechanics of ATP Production:
Proton Gradients, ATP Synthase, Inhibitors, Uncouplers, and Shuttles
We've established that the Electron Transport Chain (ETC) is a sophisticated system for moving electrons and generating energy. Now, let's connect the dots to how that energy is actually converted into ATP, how the system can be sabotaged, and how electrons from outside the mitochondria get into this vital pathway.
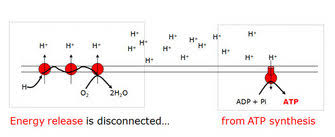
I. The Proton Gradient: The Engine's Potential Energy
Imagine a hydroelectric dam. The water held behind the dam represents potential energy. In our cellular power plant, this "potential energy" is stored in a proton gradient.
-
The Inner Mitochondrial Membrane: A Selective Barrier: The inner mitochondrial membrane is a highly specialized barrier. It's impermeable to protons (
H⁺) and hydroxyl ions (OH⁻). This impermeability is absolutely critical.
Analogy: Think of it as a perfectly sealed wall in our dam. If the wall were leaky, you couldn't build up pressure. -
Proton Pumping: Building the Pressure: As electrons move through the ETC (at Complexes I, III, and IV), energy is released. This energy is used to actively pump protons (
H⁺) from the mitochondrial matrix into the intermembrane space.
Analogy: This is like giant pumps moving water from the riverbed up into the reservoir behind the dam. -
The Result: An Electrochemical Gradient: This pumping action creates two things:
- A chemical gradient: There are now many more protons in the intermembrane space than in the matrix.
- An electrical gradient: Protons are positively charged, so the intermembrane space becomes positively charged relative to the matrix.
- The Purpose: This gradient is developed due to the electron flow in the ETC. Its sole purpose is to drive the synthesis of ATP from ADP and Pi.
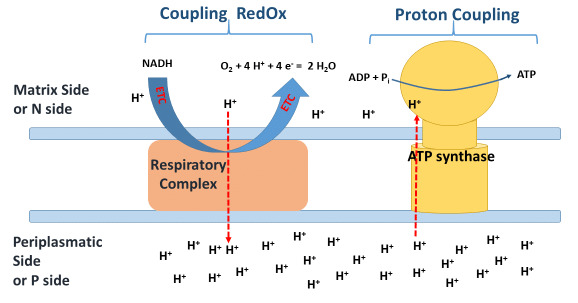
II. Coupling of Electron Transport and ATP Synthesis
The core principle is how the electron flow is coupled to ATP synthesis.
- The "Coupling" Concept: Imagine two gears meshing together. One gear (electron transport) turns the other gear (ATP synthesis). They are linked. Protons (
H⁺) are pumped from the matrix into the intermembrane space, creating the proton gradient. Then, ATP synthase allows protons to flow back into the matrix, powering the enzyme to produce ATP. This is the essence of chemiosmotic coupling.
III. Outline of Chemiosmotic Hypothesis
This is the accepted model for how oxidative phosphorylation works.
- The Core Idea: Energy from the ETC (electron flow) creates a proton gradient across the inner mitochondrial membrane. The potential energy stored in this gradient is then used by ATP synthase to make ATP.
- Analogy: It's the entire hydroelectric dam in one picture. The electron "river" (ETC) powers the pumps to create the proton "reservoir" (intermembrane space), and the water flowing back through the "turbines" (ATP synthase) generates "electricity" (ATP).
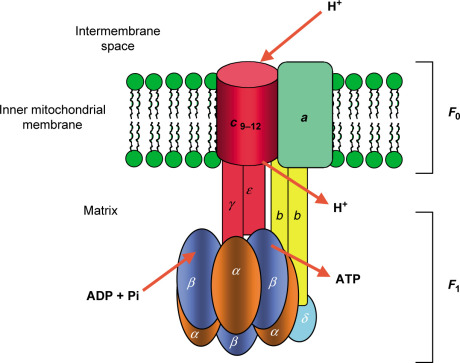
IV. Enzyme System for ATP Synthesis: ATP Synthase
The star of the show is a remarkable molecular machine called ATP synthase.
- The Master Enzyme: ATP synthase (also called Complex V) is an enzyme in the inner mitochondrial membrane that utilizes the proton gradient for the synthesis of ATP. It's also known as ATPase because it can hydrolyze ATP to ADP and Pi.
-
Structure: ATP synthase has two functional parts:
- F₀ (F-zero) component: Embedded in the membrane; it's the proton channel.
- F₁ (F-one) component: Protrudes into the matrix; this is the catalytic head where ATP is made.
- Structure of Mitochondrial ATP Synthase:
- The entire enzyme is a single F₀F₁ complex.
- The F₀ domain contains a rotating ring of 'c' subunits. A central stalk (γ subunit) is attached to this ring and extends into the F₁ head.
- The F₁ domain is the catalytic part, a sphere-like structure made of three α subunits and three β subunits. The β subunits are where ATP synthesis occurs.
- The Binding Change Model: This is the ingenious mechanism by which ATP synthase works. It's a rotating molecular motor.
- Proton Flux Drives Rotation: The flow of protons through the F₀ channel causes the 'c' subunit ring and the central γ stalk to rotate.
- Conformational Changes: This rotation causes the three β subunits in the F₁ head to change their shape sequentially. There are three conformations:
- Loose (L) conformation: Binds ADP and Pi loosely.
- Tight (T) conformation: Binds ADP and Pi tightly and catalyzes the formation of ATP.
- Open (O) conformation: Releases the newly synthesized ATP.
- The Cycle: Each β subunit cycles through L → T → O conformations. For every full rotation of the γ subunit, three ATP molecules are produced.
V. Summary of Energy Production
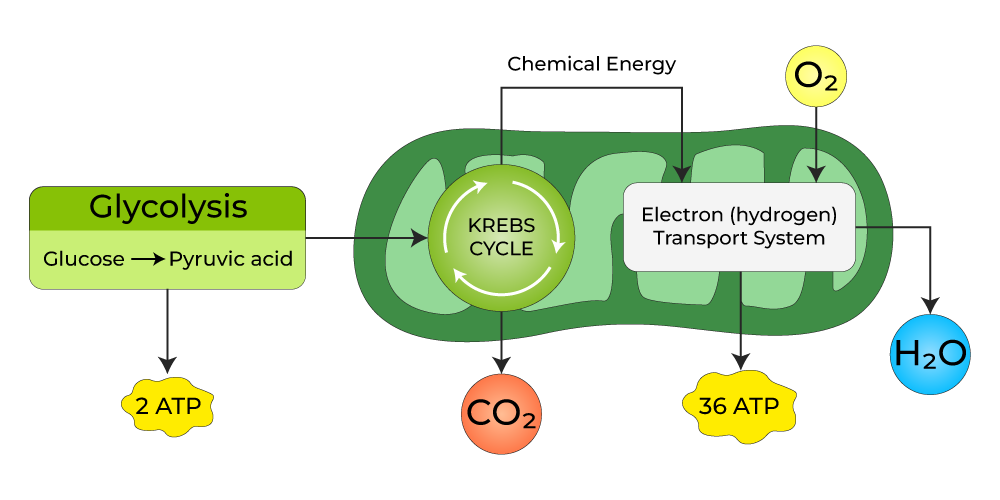
This integrates all the major metabolic pathways:
- Glycolysis (Cytoplasm): Breaks down glucose into pyruvate, producing a small amount of ATP and NADH.
- Krebs Cycle / Citric Acid Cycle (Mitochondrial Matrix): Further oxidizes pyruvate, producing more NADH, FADH₂, and some GTP (converted to ATP).
- Electron Transport Chain & Oxidative Phosphorylation (Inner Mitochondrial Membrane): The grand finale, where the vast majority of ATP is produced using the NADH and FADH₂ from the earlier steps.
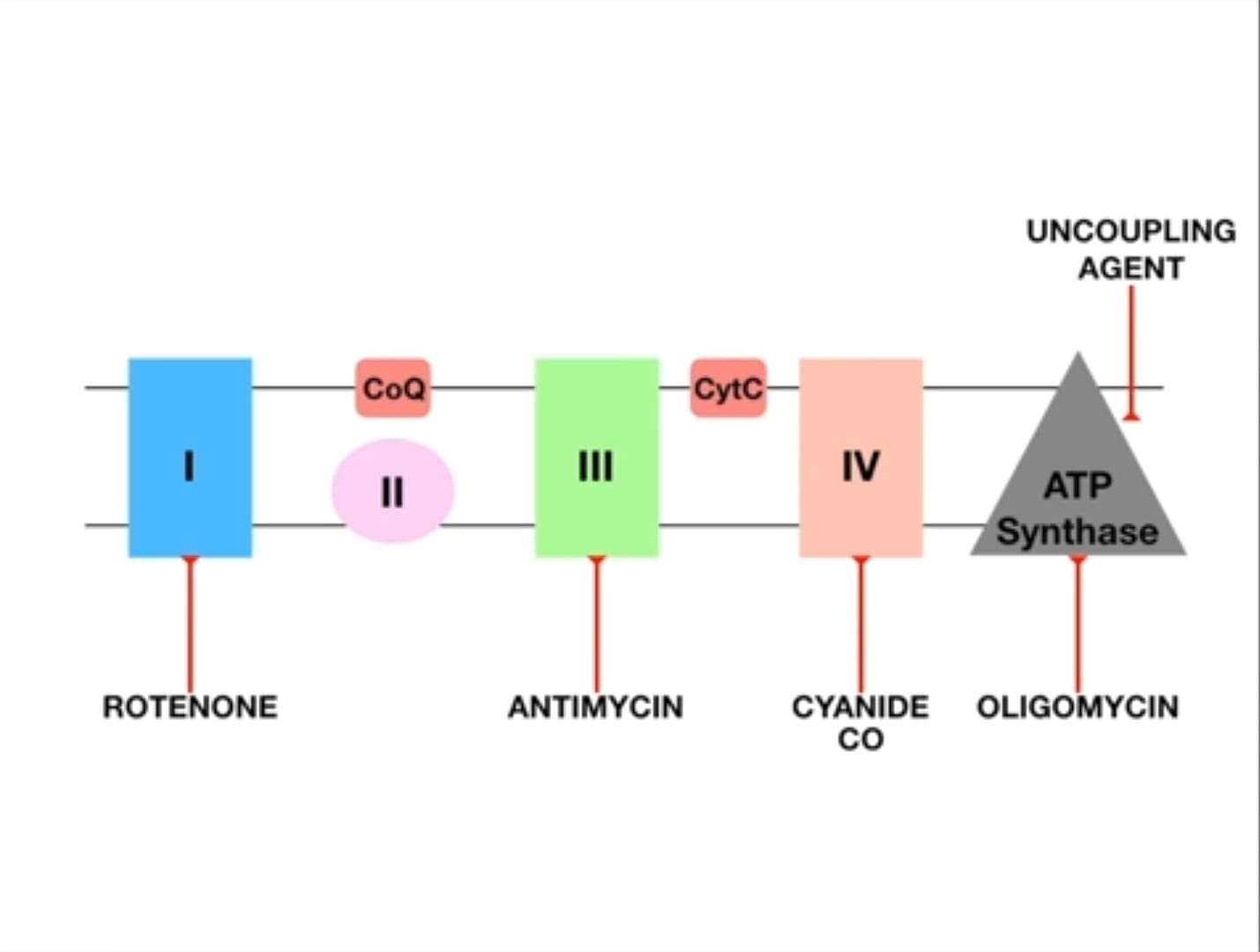
VI. Site-Specific Inhibitors of the ETC
The ETC is so critical that interfering with it can be deadly. Various compounds act as inhibitors, blocking specific points in the chain.
- Mechanism of Inhibition: Inhibitors bind to a component of the ETC, blocking electron flow.
- Upstream Accumulation: Components before the blockage accumulate electrons (become reduced).
- Downstream Oxidation: Components after the blockage become oxidized (lack electrons).
- No ATP: Blocking the ETC immediately halts ATP production.
Examples of Inhibitors:
-
Inhibitors of NADH Dehydrogenase (Complex I):
- Rotenone (pesticide), Amytal (barbiturate), Piericidin A (antibiotic).
- Effect: Blocks electron transfer from Complex I to CoQ.
-
Inhibitors of Cytochrome b-c₁ complex (Complex III):
- Antimycin A (antibiotic), BAL (British Anti-Lewisite).
- Effect: Blocks electron transfer from Complex III to cytochrome c.
-
Inhibitors of Cytochrome Oxidase (Complex IV): These are particularly dangerous.
- Carbon Monoxide (CO): Binds tightly to the Fe²⁺ in cytochrome a₃, preventing oxygen from binding.
- Cyanide (CN⁻): Binds tightly to the Fe³⁺ in cytochrome a₃.
- Azide: Also inhibits Complex IV.
- Effect: Blocks electron transfer to oxygen, effectively shutting down the entire ETC. This is why cyanide poisoning causes rapid death due to histotoxic hypoxia.
VII. Inhibitors of Oxidative Phosphorylation (Uncouplers)
Unlike inhibitors that stop electron transport, uncouplers allow electron transport to continue, but they disconnect it from ATP synthesis.
-
The Uncoupling Effect: Normally, electron transport is tightly "coupled" with ATP synthesis. Uncouplers disconnect these two processes.
Analogy: Imagine a dam where the pumps (ETC) are still moving water (protons), but the turbine (ATP synthase) is no longer connected to the generator. The water flows, but no electricity is made. -
Mechanism: Uncouplers are typically lipophilic weak acids that carry protons across the inner mitochondrial membrane, bypassing ATP synthase. They make the membrane permeable to protons.
Analogy: They poke holes in our dam wall. Protons leak back without passing through ATP synthase. -
The Result:
- Electron transport continues (often at an accelerated rate).
- No ATP is produced.
- Energy is released as heat.
Examples of Uncouplers:
- 2,4-Dinitrophenol (DNP): A notorious uncoupler once used as a weight-loss drug, leading to dangerous hyperthermia (overheating).
- Valinomycin, gramicidin, nigericin (antibiotics).
- Pentachlorophenol (PCP), FCCP, dicumarol, aspirin (at high doses).
Physiological Uncouplers:
- Some uncoupling can occur naturally in the body.
- Thermogenin (UCP1): A protein found in brown adipose tissue (BAT).
- Bilirubin: Also identified as an uncoupler.
Significance of Uncoupling:
- Heat Generation: Uncoupling is crucial for maintaining body temperature, especially in hibernating animals and newborns.
- Brown Adipose Tissue (BAT): This specialized tissue is rich in mitochondria and thermogenin. When activated, BAT uncouples oxidative phosphorylation, leading to rapid heat production without shivering.
VIII. Transport of Reducing Equivalents – Shuttle Pathways
Glycolysis happens in the cytoplasm, producing NADH. The ETC is in the mitochondria. The inner mitochondrial membrane is impermeable to NADH. So, how do these cytosolic electrons get into the ETC? Through shuttle systems!
- The Problem: NADH produced in the cytosol cannot directly cross the inner mitochondrial membrane.
- The Solution: Shuttle systems transport the "reducing equivalents" (electrons) from cytosolic NADH into the mitochondria.
Analogy: It's like having a cargo ship in a port (cytosol), but the factory (mitochondria) is inland. You need smaller trucks (shuttle systems) to deliver the goods.
1. Glycerol 3-Phosphate Shuttle:
- Mechanism:
- In the cytosol, an enzyme oxidizes NADH to NAD⁺, using the electrons to reduce dihydroxyacetone phosphate (DHAP) to glycerol 3-phosphate.
- Glycerol 3-phosphate moves to the inner mitochondrial membrane.
- Here, a different enzyme oxidizes glycerol 3-phosphate back to DHAP, transferring the electrons to FAD, forming FADH₂.
- This FADH₂ then delivers its electrons to Coenzyme Q in the ETC.
- ATP Yield: Because these electrons enter the ETC at the level of FADH₂ (bypassing Complex I), they result in the synthesis of ~2 ATPs per cytosolic NADH.
2. Malate-Aspartate Shuttle:
This shuttle is more complex but more efficient, primarily active in the heart and liver.
- Mechanism:
- In the cytosol, oxaloacetate (OAA) accepts electrons from NADH to become malate.
- Malate enters the mitochondrial matrix.
- Inside the matrix, an enzyme oxidizes malate back to OAA, regenerating NADH inside the matrix.
- This mitochondrial NADH can now directly enter Complex I of the ETC.
- (A complex cycle involving aspartate is used to return the OAA back to the cytosol).
- ATP Yield: Since the electrons are delivered to NADH within the matrix, they enter at Complex I, yielding ~3 ATPs per cytosolic NADH.
Biochemistry: Biological Oxidation & ETC
Test your knowledge with these 40 questions.
Biological Oxidation Exam
Question 1/40
Exam Complete!
Here are your results, .
Your Score
38/40
95%