Lipids Biochemistry: Fats or what?
LIPIDS
Unlike carbohydrates and proteins, which are defined by repeating monomeric units (monosaccharides, amino acids, respectively) and specific functional group chemistry, lipids are not polymers in the classical sense, nor are they defined by a single, specific functional group.
Instead, lipids are defined primarily by a crucial physical property: their hydrophobic nature.
Primary Defining Characteristic:
Hydrophobicity
Lipids are a group of organic compounds characterized by their insolubility in water. This is their most distinguishing and unifying feature.
- Molecular Basis of Insolubility: This insolubility stems from their molecular structure, which is predominantly composed of nonpolar hydrocarbon regions. These regions consist primarily of carbon-carbon (C-C) and carbon-hydrogen (C-H) bonds, which have very similar electronegativities, leading to an even distribution of electrons and thus no significant partial charges.
- The Hydrophobic Effect: Water, being a highly polar solvent, forms extensive and strong hydrogen bonds with itself, creating a highly ordered network. Nonpolar molecules, lacking the partial charges or hydrogen-bonding capabilities, cannot participate in these favorable interactions. Consequently, water molecules tend to "exclude" or push nonpolar molecules together to minimize the disruption to their hydrogen-bonding network and reduce the unfavorable surface area contact between water and nonpolar substances. This phenomenon is known as the hydrophobic effect, and it is the primary driving force for lipid aggregation (e.g., membrane formation, fat droplet formation) in aqueous environments.
- Solubility in Organic Solvents: Conversely, lipids are readily soluble in nonpolar (or weakly polar) organic solvents, such as diethyl ether, chloroform, benzene, and acetone. This "like dissolves like" principle is fundamental to lipid chemistry and is often exploited for their extraction and purification from biological tissues.
Elemental Composition:
Lipids are primarily composed of carbon (C), hydrogen (H), and a smaller proportion of oxygen (O) compared to carbohydrates. While carbohydrates have a typical empirical formula of (CH2O)n, lipids have significantly fewer oxygen atoms relative to carbon and hydrogen.
Other Elements: Some lipids also contain other elements critical for their specific functions:
- Phosphorus (P): Found in phospholipids, which are essential components of biological membranes. The phosphate group contributes to the hydrophilic head of these molecules.
- Nitrogen (N): Found in certain phospholipids (e.g., phosphatidylcholine, phosphatidylethanolamine) and sphingolipids (e.g., sphingomyelin, gangliosides), often within the hydrophilic head groups.
Energy Density:
Lipids are renowned as energy-dense molecules. They store more energy per gram than carbohydrates or proteins.
- High Energy Content: This high energy yield (approximately 9 kcal/gram or 37 kJ/gram) is a direct consequence of their highly reduced (less oxidized) state. The many C-H bonds in their hydrocarbon chains contain a large amount of potential energy that can be released upon oxidation (metabolism). This contrasts with carbohydrates and proteins, which yield about 4 kcal/gram (17 kJ/gram) and contain more oxygen, indicating a more oxidized state.
Biological Functions of Lipids
a. Energy Storage (Long-Term)
Triglycerides (fats and oils) represent the most efficient and concentrated form of energy storage in living organisms.
- Superior Energy Yield: As noted, they yield approximately 9 kcal (37 kJ) of energy per gram upon complete oxidation, more than double that of carbohydrates or proteins. This makes them ideal for long-term energy reserves.
- Anhydrous Storage: Their hydrophobic nature is a significant advantage for storage. Triglycerides are stored in an anhydrous (water-free) form. In contrast, carbohydrates like glycogen are highly hydrated, binding about 2 grams of water per gram of glycogen. Storing energy as fat significantly saves considerable space and weight, which is particularly crucial for mobile organisms (animals) and for seeds.
- Examples:
- Animals: Adipose tissue (fat cells) in mammals stores triglycerides, providing insulation and cushioning in addition to energy reserves.
- Plants: Oils are stored in seeds (e.g., sunflower, olive, peanut) to provide energy for germination and seedling growth.
b. Structural Components of Biological Membranes
Phospholipids and glycolipids are the fundamental building blocks of all biological membranes, defining the boundaries of cells and their internal organelles.
- Amphipathic Nature: These lipids possess a unique amphipathic (or amphiphilic) nature, meaning they have both a hydrophilic ("water-loving") head group and hydrophobic ("water-fearing") hydrocarbon tails. The head typically contains a phosphate or sugar, while the tails consist of two long fatty acid chains.
- Lipid Bilayer Formation: In an aqueous environment, this property drives their spontaneous self-assembly into a lipid bilayer. The hydrophobic tails orient towards the interior, away from water, while the hydrophilic heads face outwards. This forms a stable, fluid barrier that is selectively permeable.
- Cholesterol's Role: Cholesterol, a type of steroid lipid, plays a crucial role in regulating the fluidity and integrity of animal cell membranes. It inserts into the bilayer, modulating membrane permeability and preventing the membrane from becoming too rigid or too fluid.
c. Signaling Molecules
Many lipids act as potent signaling molecules, functioning as hormones or intracellular messengers that regulate a vast array of physiological processes.
- Steroid Hormones: Derived from cholesterol, they act as long-distance messengers. Examples include estrogen, progesterone, testosterone (reproduction), cortisol (metabolism), and aldosterone (salt balance).
- Eicosanoids: Potent local signaling molecules derived from fatty acids. Examples include prostaglandins (inflammation, pain), thromboxanes (blood clotting), and leukotrienes (allergic responses).
- Lipid-derived Second Messengers: Crucial for intracellular signaling. Examples include Diacylglycerol (DAG) and Inositol trisphosphate (IP₃), which are derived from membrane phospholipids and trigger various cellular responses.
d. Vitamins and Coenzymes
Several essential vitamins are lipid-soluble (fat-soluble), meaning they are absorbed, transported, and stored in the body along with dietary fats.
- Vitamin A (Retinol): Essential for vision, cell growth, and immune function.
- Vitamin D (Calciferol): Functions as a hormone precursor, regulating calcium for bone health.
- Vitamin E (Tocopherols): A powerful antioxidant that protects cell membranes from oxidative damage.
- Vitamin K: Essential for blood clotting.
- Ubiquinone (Coenzyme Q): A lipid-soluble electron carrier in mitochondria, vital for ATP production.
e. Insulation and Protection
Lipids provide vital physical protection and thermal regulation in organisms.
- Thermal Insulation: Adipose tissue forms a subcutaneous layer (e.g., blubber in marine mammals) that provides excellent thermal insulation, maintaining stable body temperature.
- Mechanical Cushioning: Adipose tissue also acts as a mechanical cushion, absorbing physical shocks around vital organs like the kidneys and heart.
- Protective Coatings (Waxes): Waxes are highly hydrophobic and form water-repellent coatings on plant leaves (cuticle), insect exoskeletons, and animal fur/feathers to prevent water loss and protect from pathogens.
f. Buoyancy
In aquatic animals, lipid stores can significantly contribute to buoyancy, helping them to float or maintain their depth in water without expending excessive energy.
- Lower Density: Fats and oils are less dense than water. By accumulating large quantities of lipids (e.g., in blubber or oil-rich livers), aquatic organisms can achieve neutral or positive buoyancy.
- Examples: Marine mammals (whales, seals) use blubber; sharks use their large, oil-rich livers for buoyancy.
Classification of Lipids:
Given their structural differences, lipids are classified into several major categories based on their chemical structure and precursor molecules.
- Fatty Acids: The simplest form of lipids and often serve as the building blocks for many other complex lipids.
- Triglycerides (Triacylglycerols): Esters of glycerol and three fatty acids.
- Phospholipids: Derived from glycerol (glycerophospholipids) or sphingosine (sphingolipids).
- Glycolipids: Contain a carbohydrate moiety attached to a lipid (often sphingosine or glycerol).
- Steroids: Characterized by a distinctive four-ring core structure (steroid nucleus).
- Waxes: Esters of long-chain fatty acids and long-chain alcohols.
- Eicosanoids: Derived from C20 polyunsaturated fatty acids (like arachidonic acid).
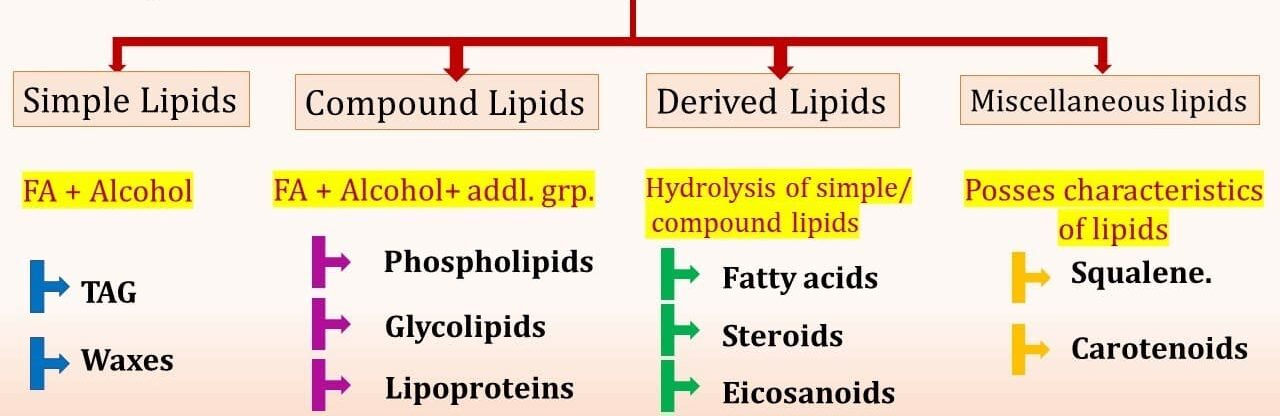
Table summarizing the major classes of lipids.
| Category | Key Characteristics & Components | Examples (as per image & additional) |
|---|---|---|
| I. Simple Lipids | Esters of Fatty Acids (FA) with various Alcohols | Triglycerides (TAG), Waxes |
| II. Compound Lipids | Esters of FA + Alcohol + Additional groups | Phospholipids, Glycolipids, Lipoproteins |
| III. Derived Lipids | Products of hydrolysis of simple/compound lipids | Fatty Acids, Steroids, Eicosanoids, Glycerol |
| IV. Miscellaneous Lipids | Possess characteristics of lipids (hydrophobicity) but don't fit other categories | Squalene, Carotenoids, Vitamins A, D, E, K |
Note: This classification system is common in biochemistry but can vary slightly across different textbooks. Lipoproteins, while containing lipids, are often classified as complex molecules due to their protein component, facilitating lipid transport.
I. Simple Lipids:
We will start by detailing the building blocks and then move into the simple lipids.

Fatty Acids
Fatty acids are the simplest form of lipids and often serve as the primary building blocks for many other more complex lipids, such as triglycerides, phospholipids, and waxes. During digestion, fats (triglycerides) are broken down into fatty acids and glycerol.
Structure of Fatty Acids:
A fatty acid is fundamentally a carboxylic acid with a long aliphatic (hydrocarbon) chain.
Basic Structure Components:
- A Carboxyl Group (
-COOH): This is the hydrophilic (polar) head of the fatty acid. It is acidic due to the readily ionizable hydrogen. At physiological pH (around 7.4), this group is typically ionized, existing as a carboxylate group (-COO⁻), which contributes significantly to its polar and hydrophilic nature. - A Hydrocarbon Chain: This is the hydrophobic (nonpolar) tail, composed solely of carbon and hydrogen atoms (
C-Hbonds). The length of this chain and the presence or absence of double bonds are crucial determinants of the fatty acid's physical and chemical properties.
General Formula: R-COOH, where 'R' represents the hydrocarbon chain.
Chain Length Variations:
Fatty acids commonly found in biological systems usually have an even number of carbon atoms, ranging from 4 to 28 carbons. This even number is a consequence of their biosynthesis from 2-carbon units (acetyl-CoA).
- Short-chain fatty acids (SCFAs): 2-6 carbons (e.g., acetate (
C2:0), butyrate (C4:0) found in butter). - Medium-chain fatty acids (MCFAs): 8-12 carbons (e.g., caprylic acid (
C8:0) found in coconut oil). - Long-chain fatty acids (LCFAs): 14-20 carbons (e.g., palmitic acid (
C16:0), oleic acid (C18:1). These are the most common in the human diet and body). - Very long-chain fatty acids (VLCFAs): >20 carbons (e.g., lignoceric acid (
C24:0)).
Nomenclature - Methyl End and Carboxyl End:
- The carbon of the carboxyl group is designated as C-1 (the alpha (α) carbon is the one adjacent to the carboxyl group).
- The carbon chain extends from C-1.
- The last carbon atom (the one furthest from the carboxyl group) is called the omega (ω) carbon or the methyl end.
- This nomenclature is critically important, especially when discussing the position of double bonds in unsaturated fatty acids (e.g., omega-3, omega-6 fatty acids), as it indicates the position of the first double bond relative to the methyl end.
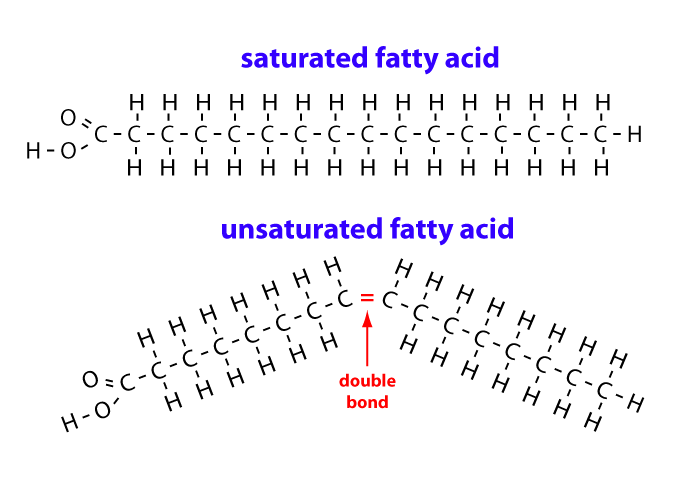
Saturated vs. Unsaturated Fatty Acids:
The presence or absence of carbon-carbon double bonds within the hydrocarbon chain is a fundamental structural feature that profoundly affects a fatty acid's physical properties, especially its melting point and fluidity.
a. Saturated Fatty Acids (SFAs):
- Structure: Contain no carbon-carbon double bonds in their hydrocarbon chain. All carbon atoms in the chain are "saturated" with the maximum number of hydrogen atoms. This lack of double bonds allows for free rotation around all
C-Csingle bonds, making the hydrocarbon chain flexible and capable of adopting an extended, relatively straight (linear), zigzag conformation. - Packing: These straight chains can pack very closely together in a highly ordered, quasi-crystalline arrangement. This tight packing allows for strong van der Waals forces (nonpolar interactions) between adjacent chains.
- Melting Point: Due to efficient packing and the cumulative strength of many van der Waals interactions, saturated fatty acids (and lipids predominantly composed of them) tend to have higher melting points. They are typically solid at room temperature (e.g., butter, animal fats, coconut oil, palm oil).
- Examples: Lauric acid (
C12:0), Myristic acid (C14:0), Palmitic acid (C16:0), Stearic acid (C18:0).
b. Unsaturated Fatty Acids (UFAs):
- Structure: Contain one or more carbon-carbon double bonds in their hydrocarbon chain. These double bonds introduce rigidity and alter the overall shape of the molecule.
- Types:
- Monounsaturated Fatty Acids (MUFAs): Contain one carbon-carbon double bond.
- Polyunsaturated Fatty Acids (PUFAs): Contain two or more carbon-carbon double bonds.
- Conformation of Double Bonds (Cis vs. Trans Isomers):
- Cis configuration: This is the predominant natural configuration. The two hydrogen atoms are on the same side of the double bond, causing a distinct, rigid "kink" or bend in the chain.
- Trans configuration: The two hydrogen atoms are on opposite sides, allowing the chain to remain relatively straight. Trans fats are uncommon in nature but are produced during industrial hydrogenation.
- Packing: The cis double bonds and their associated kinks prevent unsaturated fatty acid chains from packing as tightly as saturated chains.
- Melting Point: Less efficient packing leads to weaker van der Waals interactions and thus significantly lower melting points. Unsaturated fatty acids are typically liquid at room temperature (e.g., most vegetable oils, fish oils).
- Examples: Oleic acid (
C18:1, Δ9), Linoleic acid (C18:2, Δ9,12), α-Linolenic acid (C18:3, Δ9,12,15).
Essential Fatty Acids (EFAs):
While the human body can synthesize most fatty acids, some polyunsaturated fatty acids cannot be synthesized and must be obtained from the diet. These are termed essential fatty acids.
Two Primary Essential Fatty Acids for Humans:
- Linoleic acid (LA): An omega-6 fatty acid (first double bond at the 6th carbon from the ω end). It is the precursor for other omega-6 fatty acids like arachidonic acid, which is used to make eicosanoids that mediate inflammation. Found in vegetable oils, nuts, and seeds.
- Alpha-linolenic acid (ALA): An omega-3 fatty acid (first double bond at the 3rd carbon from the ω end). It is the precursor for longer-chain omega-3s like EPA and DHA, which are vital for many functions. Found in flaxseed oil, chia seeds, and walnuts.
Importance of Essential Fatty Acids (Elaborated):
- Maintaining healthy cell membranes: The kinks introduced by the cis double bonds in PUFAs increase the space between phospholipid molecules in the cell membrane. This prevents the membrane from becoming too rigid and maintains its fluidity, which is essential for the proper function of membrane-bound proteins like receptors, enzymes, and transport channels.
- Proper growth and development: EFAs, particularly the long-chain omega-3 fatty acid DHA (Docosahexaenoic Acid), are highly concentrated in the brain and retina. DHA is a critical structural component of neuronal and photoreceptor cell membranes, and its accumulation is vital for brain growth, synaptic development, and visual acuity, especially during fetal development and infancy.
- Synthesis of eicosanoids: The body uses arachidonic acid (derived from omega-6 LA) and EPA (derived from omega-3 ALA) to synthesize a class of potent, short-lived signaling molecules called eicosanoids (e.g., prostaglandins, thromboxanes, leukotrienes). These act as local hormones to regulate a wide range of processes, including the intensity and duration of inflammation, blood clotting, blood pressure, and immune responses.
- Nervous system function and vision: Beyond its structural role, DHA in neuronal membranes influences neurotransmitter release, signal transduction, and gene expression, all of which are fundamental to learning, memory, and overall cognitive function. Its presence in the retina is crucial for converting light into neural signals.
- Gene expression regulation: Fatty acids and their derivatives can act as signaling molecules that bind to and activate nuclear receptors (like PPARs - Peroxisome Proliferator-Activated Receptors). These receptors then function as transcription factors that regulate the expression of genes involved in lipid and carbohydrate metabolism, inflammation, and cellular differentiation.
- Skin health and integrity: EFAs are essential components of the skin's lipid barrier (in the stratum corneum). This barrier is crucial for maintaining skin hydration by preventing excessive water loss and for protecting the body from environmental insults and pathogens. A deficiency can lead to dry, scaly skin and dermatitis.
Triglycerides (Triacylglycerols)
Triglycerides, also commonly known as triacylglycerols (TAGs), are the most abundant type of lipid in the body and represent the major form of metabolic energy storage in both animals and plants. They are crucial for survival, providing a compact and highly efficient long-term energy reserve.
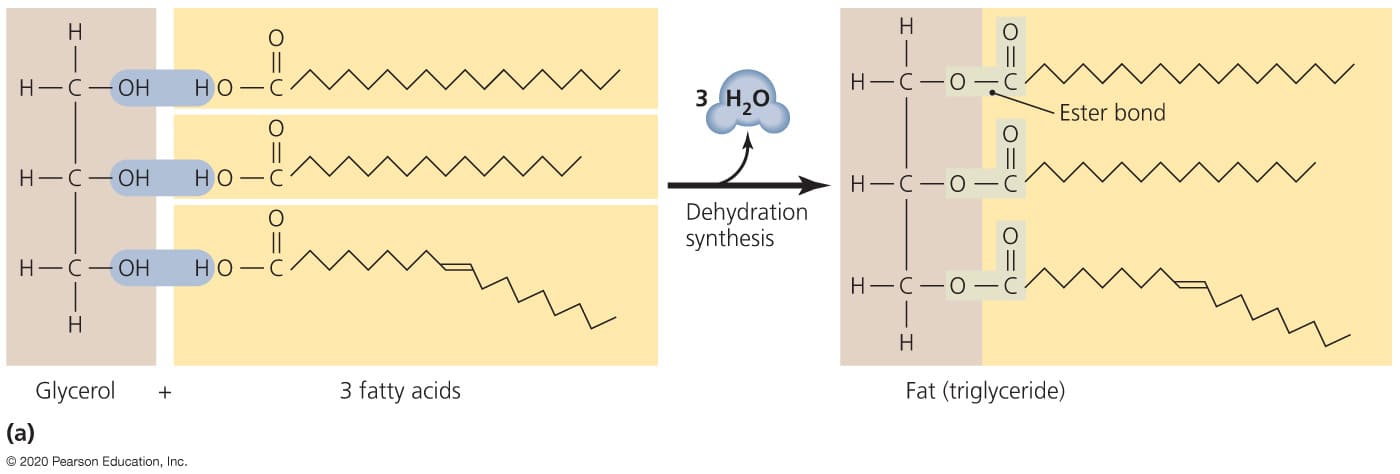
Formation of Triglycerides (Esterification / Dehydration Synthesis):
Triglycerides are formed through a process known as esterification or dehydration synthesis (also called condensation reaction). This is a chemical reaction where smaller molecules (fatty acids and glycerol) are linked together, with the simultaneous removal of water molecules.
Process Overview:
- The reaction involves the chemical union of a fatty acid molecule with an alcohol group (
-OH) from a glycerol molecule. - Specifically, a hydroxyl group (
-OH) from the glycerol molecule reacts with the hydrogen atom (H) from the carboxyl group (-COOH) of a fatty acid. - These combine to form and release a molecule of water (H2O). This removal of water facilitates the formation of a strong covalent bond, an ester bond, between the glycerol and the fatty acid.
Resulting Molecules (based on the number of fatty acid attachments):
- Monoglyceride (Monoacylglycerol): If only one fatty acid is linked to the glycerol backbone. (Glycerol + 1 Fatty Acid)
- Diglyceride (Diacylglycerol): If two fatty acids are linked to the glycerol backbone. (Glycerol + 2 Fatty Acids) Diglycerides often play roles in cell signaling.
- Triglyceride (Triacylglycerol): If three fatty acids are linked to the glycerol backbone. (Glycerol + 3 Fatty Acids) This is the primary storage form.
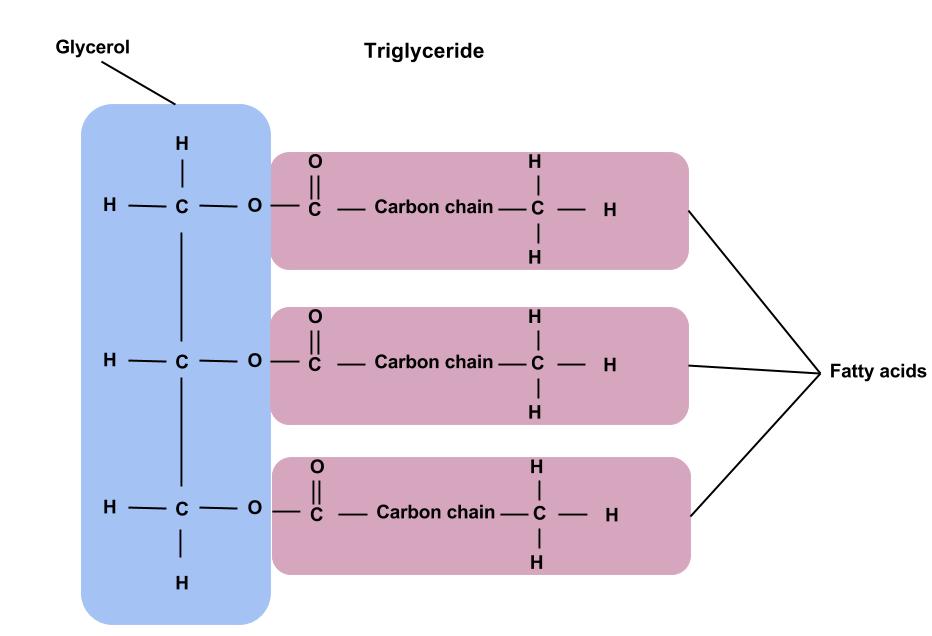
Structure: Glycerol Backbone Esterified to Three Fatty Acids
Triglycerides are defined structurally as esters of glycerol and three fatty acids.
- Glycerol Backbone:
- Glycerol is a simple, three-carbon alcohol (chemically named propane-1,2,3-triol).
- It possesses three hydroxyl (
-OH) groups, one on each of its three carbon atoms. These hydroxyl groups are the specific sites where the fatty acids attach, forming ester bonds.
- Ester Bonds:
- Each of the three hydroxyl groups on the glycerol molecule forms an ester bond with the carboxyl group (
-COOH) of a fatty acid. - An ester bond is a strong covalent link formed between an alcohol and a carboxylic acid, with the elimination of a molecule of water.
- Therefore, during the complete synthesis of one triglyceride molecule, three molecules of water are released (one for each fatty acid esterified).
- Each of the three hydroxyl groups on the glycerol molecule forms an ester bond with the carboxyl group (
- Fatty Acid Variability:
- The three fatty acids esterified to the glycerol backbone in a single triglyceride molecule can be the same or, more commonly, different. This variability can occur in terms of chain length and degree of saturation.
- This significant variability contributes to the vast diversity of triglycerides found in nature, giving different fats and oils their unique physical properties (e.g., solid butter vs. liquid olive oil).
- Simple Triglyceride: If all three fatty acids are identical (e.g., tristearin).
- Mixed Triglyceride: If the three fatty acids are different. Most naturally occurring triglycerides are mixed triglycerides.
Function: Primary Form of Energy Storage
Triglycerides serve as the body's principal long-term energy reserve due to several highly advantageous properties:
- High Energy Yield: As previously discussed, triglycerides are highly reduced molecules, meaning they have a large number of
C-Hbonds and relatively few oxygen atoms. This chemical structure translates into a very high energy content. Complete oxidation of the fatty acids within triglycerides yields approximately 9 kcal/g (37 kJ/g), which is more than double the energy yield from carbohydrates or proteins (both approximately 4 kcal/g). - Anhydrous Storage: Due to their nonpolar and highly hydrophobic nature, triglycerides are stored in an anhydrous (water-free) state within cells, primarily in specialized cells called adipocytes (fat cells). In stark contrast, glycogen is highly hydrated, binding about 2 grams of water per gram. Storing energy as triglycerides is therefore significantly more compact and lighter.
- Unlimited Storage Capacity: Unlike glycogen stores, which are relatively limited, the body has an almost unlimited capacity to store triglycerides in adipose tissue, allowing for energy reserves for extended periods.
- Insulation and Protection: Adipose tissue, largely composed of triglycerides, also performs crucial roles beyond energy storage:
- Thermal Insulation: Located beneath the skin (subcutaneous fat), it forms an insulating layer that helps to prevent heat loss (e.g., the thick layer of blubber in marine mammals).
- Mechanical Protection/Cushioning: Adipose tissue surrounds and cushions vital organs such as the kidneys and heart, acting as a shock absorber.
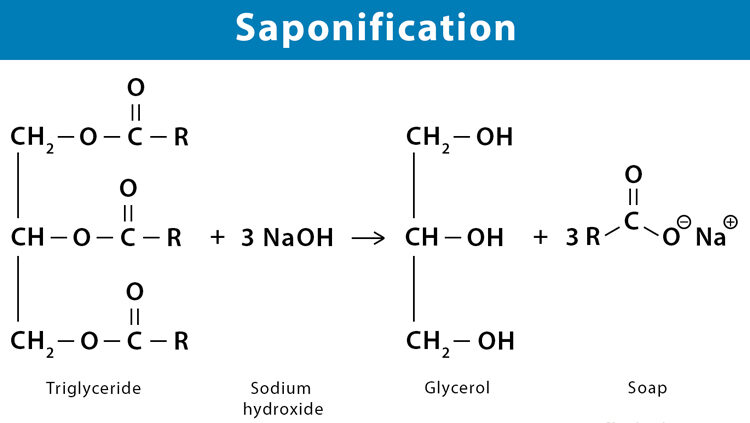
Saponification:
Hydrolysis of Triglycerides with a Strong Base (Soap Formation)
Saponification is a classic chemical reaction involving the alkaline hydrolysis of esters, specifically the hydrolysis of triglycerides using a strong base (such as sodium hydroxide, NaOH, or potassium hydroxide, KOH). This process is historically significant as it is the traditional method for making soap.
Process:
- When triglycerides are heated with a strong base, the ester bonds linking the fatty acids to the glycerol backbone are cleaved (hydrolyzed).
- This reaction yields two primary products: glycerol and the alkali metal salts of the fatty acids.
- These alkali metal salts of fatty acids are precisely what we refer to as soap.
Mechanism (Simplified):
- The strong base provides hydroxyl ions (
OH⁻), which act as a nucleophile. - The hydroxyl ion attacks the carbonyl carbon of the ester bond within the triglyceride molecule.
- This attack leads to the breakage of the ester bond, releasing the glycerol backbone and the carboxylate group (the ionized form of the fatty acid).
- The metal ion from the base (e.g.,
Na⁺fromNaOH) then associates with the negatively charged carboxylate group, forming the fatty acid salt (e.g., sodium stearate, a common soap component).
Properties of Soap:
- Soap molecules are inherently amphipathic. This means they possess:
- A long nonpolar hydrocarbon tail (derived from the fatty acid), which is hydrophobic (water-fearing) and lipophilic (fat-loving).
- A polar, charged head group (the carboxylate group,
-COO⁻), which is hydrophilic (water-loving).
- This amphipathic nature is what allows soap to function effectively as a cleaning agent. The nonpolar tails can interact with and dissolve greasy, nonpolar dirt and oil. Simultaneously, the polar heads interact strongly with water. This enables soap to emulsify fats and oils, breaking them down into tiny droplets (micelles) that are suspended in water and can then be washed away.
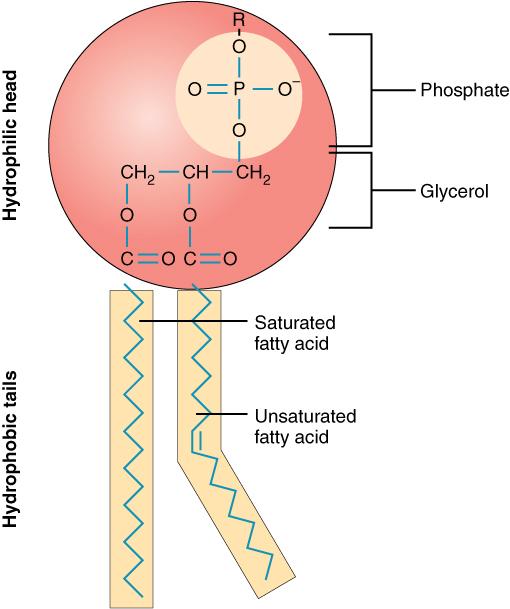
Phospholipids
Phospholipids are arguably the most important class of lipids due to their central and indispensable role in forming the structural basis of all biological membranes (e.g., plasma membrane, mitochondrial membranes, endoplasmic reticulum, etc.). Their unique amphipathic nature makes them perfectly suited for this fundamental biological function.
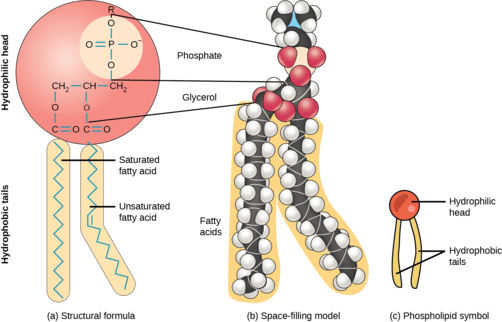
Structure:
Glycerol Backbone, Two Fatty Acids, and a Phosphate Group
The defining characteristic feature of a phospholipid is the presence of a phosphate group. Most phospholipids found in cellular membranes are derived from glycerol, and are thus called glycerophospholipids (or phosphoglycerides).
Glycerophospholipid Structure:
A typical glycerophospholipid has a distinct architecture:
- Glycerol Backbone: Similar to triglycerides, a three-carbon glycerol molecule serves as the structural backbone. The carbons are typically designated C1, C2, and C3 (or sn-1, sn-2, sn-3 in stereospecific numbering).
- Two Fatty Acids: Instead of three (as in triglycerides), two fatty acids are esterified to the first (
C1) and second (C2) carbons of the glycerol backbone. These two fatty acid chains are typically long and hydrophobic, forming the nonpolar tails of the phospholipid.- Often, the fatty acid at
C1is saturated, and the fatty acid atC2is unsaturated. This arrangement, particularly the kink introduced by the unsaturated fatty acid, is crucial for maintaining appropriate membrane fluidity.
- Often, the fatty acid at
- Phosphate Group: A highly polar and negatively charged phosphate group (
PO₄³⁻) is esterified to the third (C3) carbon of the glycerol backbone. This phosphate group, being ionized at physiological pH, contributes significantly to the polar and hydrophilic nature of one end of the molecule. - Head Group (Polar Group): In most phospholipids, the phosphate group is further esterified to a small, polar or charged molecule, which is referred to as the head group. The nature of this head group is critical as it determines the specific identity and properties of the phospholipid. Common head groups include:
- Choline: Forms Phosphatidylcholine (Lecithin).
- Ethanolamine: Forms Phosphatidylethanolamine.
- Serine: Forms Phosphatidylserine.
- Inositol: Forms Phosphatidylinositol, important in cell signaling.
- Hydrogen (H): If no additional head group is attached, it forms Phosphatidic acid, a crucial precursor.
Amphipathic Nature:
The combination of a highly polar, charged head group (phosphate + additional group) and two long, nonpolar hydrocarbon tails gives phospholipids their defining amphipathic (or amphiphilic) character.
- Hydrophilic Head: The polar head group (glycerol-phosphate-head group) is "water-loving" and readily interacts with the aqueous environment.
- Hydrophobic Tails: The two fatty acid tails are "water-fearing" and tend to avoid water, preferring to interact with other nonpolar molecules.
This dual nature is the basis for their spontaneous self-assembly into structures like lipid bilayers in aqueous environments.
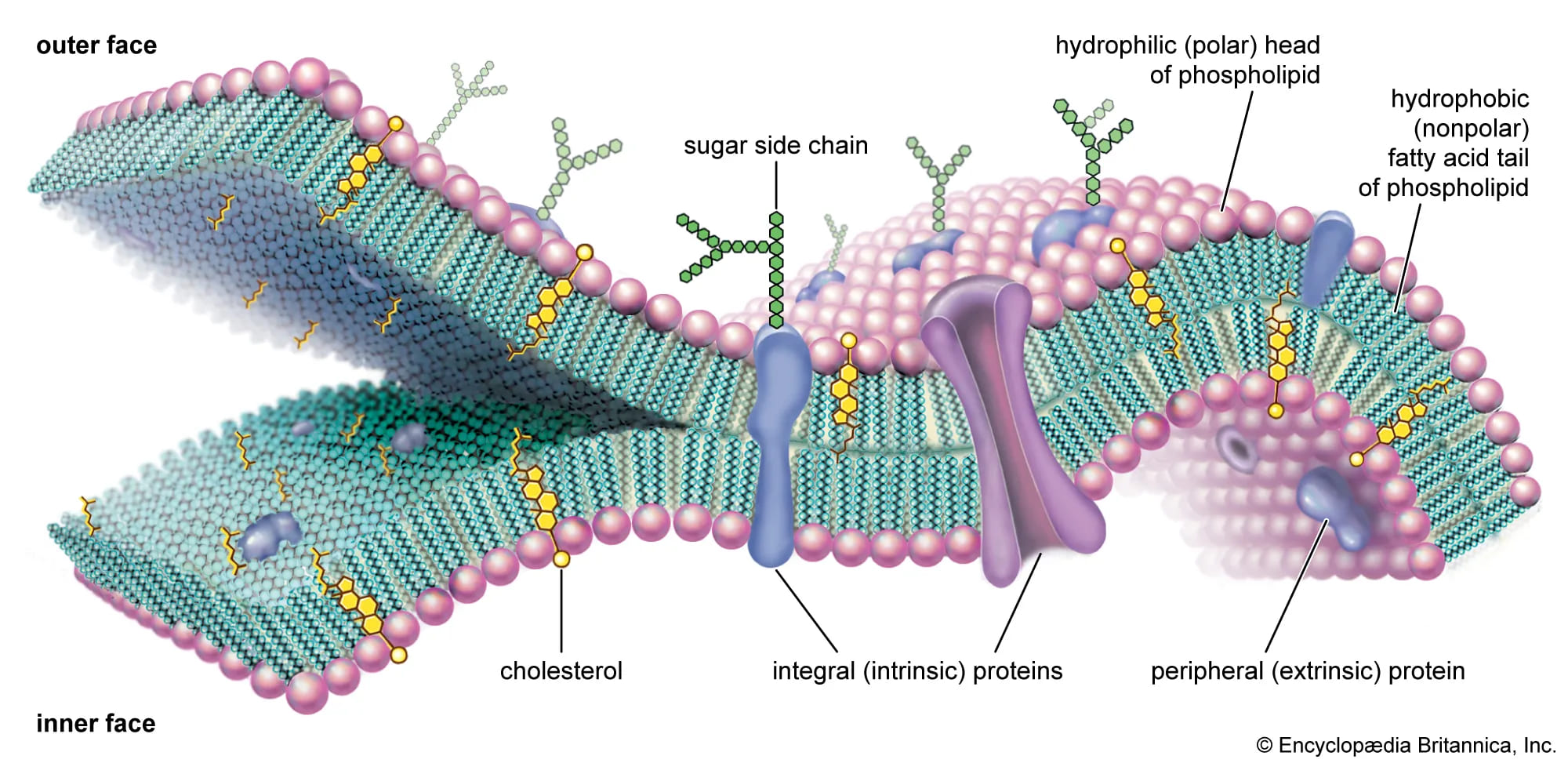
Biological Role: Formation of Lipid Bilayers
In an aqueous environment, phospholipids spontaneously arrange themselves into a lipid bilayer. This fundamental structure forms the backbone of all biological membranes.
- Bilayer Formation: The hydrophobic tails orient inward, away from the water, forming a nonpolar core. The hydrophilic heads orient outward, facing the aqueous extracellular and intracellular environments.
- Permeability Barrier: The hydrophobic core of the bilayer acts as a highly effective barrier to the passage of most polar molecules, ions, and large macromolecules, thus maintaining cellular integrity and compartmentation.
- Fluid Mosaic Model: The lipid bilayer is not a static structure but a dynamic one, as described by the fluid mosaic model. Lipids and many proteins can move laterally within the plane of the membrane, providing flexibility and enabling various cellular processes.
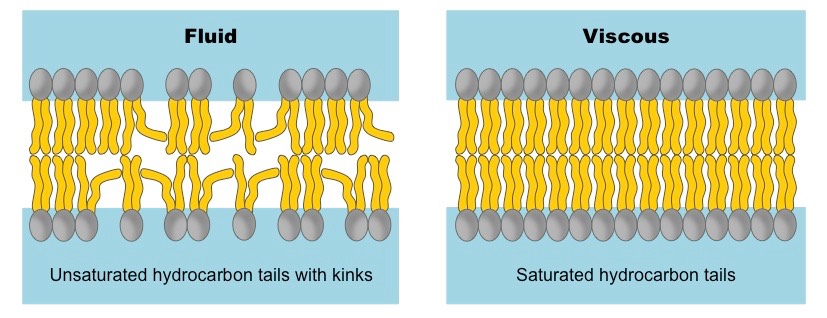
Membrane Fluidity:
Role of Saturated vs. Unsaturated Fatty Acids and Cholesterol
Biological membranes are not static, rigid structures; they are fluid, dynamic entities that allow for the lateral movement of lipids and embedded proteins within the plane of the membrane. This fluidity is essential for membrane function (e.g., protein activity, cell signaling, cell division, membrane fusion). Several key factors influence this crucial property:
Fatty Acid Composition of Phospholipids:
- Unsaturated Fatty Acids: The presence of cis double bonds in unsaturated fatty acid tails introduces kinks or bends into the hydrocarbon chains. These kinks disrupt the tight packing of adjacent phospholipid tails, creating more space between them.
- A higher proportion of unsaturated fatty acids in the membrane leads to increased membrane fluidity (and a lower melting point for the membrane). This is vital in colder environments to prevent the membrane from becoming too rigid.
- Saturated Fatty Acids: Saturated fatty acid tails, being relatively straight, can pack tightly together with strong van der Waals interactions.
- A higher proportion of saturated fatty acids leads to decreased membrane fluidity (and a higher melting point).
- Cellular Adaptation: Cells and organisms can actively adjust the fatty acid composition of their membrane phospholipids (e.g., by increasing the proportion of unsaturated fatty acids in colder temperatures or decreasing them in warmer temperatures) to maintain appropriate membrane fluidity for optimal function.
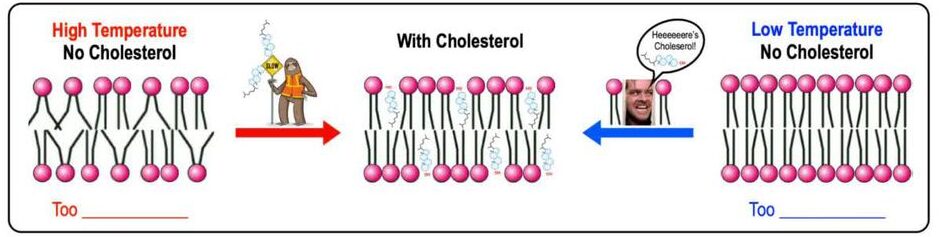
Cholesterol (in Animal Cells):
Cholesterol is a distinct type of lipid (a steroid, which will be discussed later) that is embedded within the hydrophobic core of animal cell membranes. It acts as a crucial fluidity buffer, modulating membrane fluidity across a range of temperatures:
- At High Temperatures (e.g., body temperature): Cholesterol decreases membrane fluidity. Its rigid steroid ring structure restricts the movement of phospholipid fatty acid tails, preventing the membrane from becoming too fluid, dispersed, or "leaky."
- At Low Temperatures: Cholesterol increases membrane fluidity. It intercalates between phospholipid tails, preventing them from packing too tightly together and solidifying into a gel-like state. This inhibits the membrane from becoming too rigid or brittle.
Absence in Plants and Bacteria: Plants and bacteria typically do not synthesize cholesterol. Instead, they use other sterol-like compounds to modulate membrane fluidity:
- Phytosterols: In plants (e.g., sitosterol, stigmasterol).
- Hopanoids: In bacteria, which are structurally similar to steroids.
Temperature:
- Higher Temperatures: Generally increase membrane fluidity as the kinetic energy of the lipid molecules increases, causing them to move more rapidly and pack less tightly.
- Lower Temperatures: Generally decrease membrane fluidity, causing lipids to move slower and pack more tightly, potentially leading to a more rigid, gel-like state.
Steroids
Steroids represent a class of lipids characterized by a specific four-ring structure, and they play major roles, from maintaining membrane integrity to acting as potent signaling molecules (hormones). They are derived from a common precursor, cholesterol, in animals.
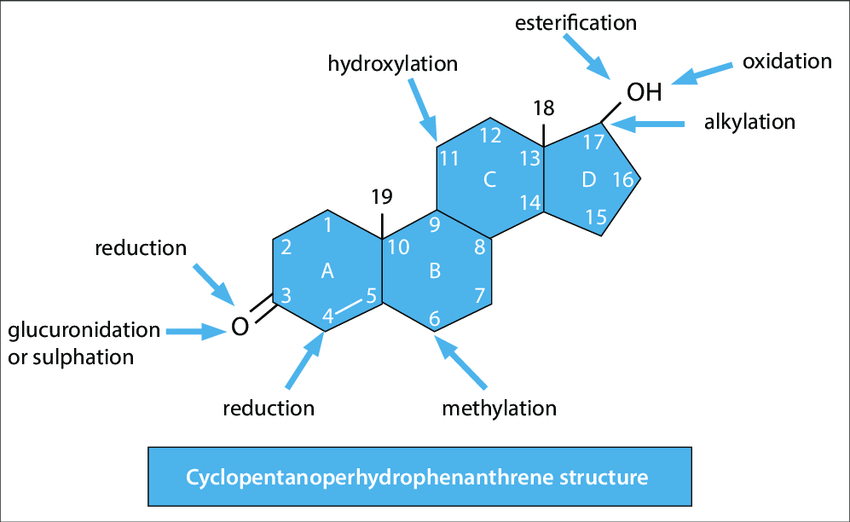
Structure: The Steroid Nucleus (Cyclopentanoperhydrophenanthrene Ring System)
The defining feature of all steroids is their characteristic core structure, known as the steroid nucleus or cyclopentanoperhydrophenanthrene ring system.
- Four Fused Rings: This nucleus consists of four fused carbon rings:
- Three six-membered cyclohexane rings (labeled A, B, and C).
- One five-membered cyclopentane ring (labeled D).
- Numbering System: The carbon atoms in the steroid nucleus are numbered systematically from 1 to 17, following established IUPAC conventions.
- Substitutions: Various steroid molecules differ by the side chains, hydroxyl groups, ketone groups, and double bonds attached to this core structure. These specific modifications dictate their diverse biological activities.
- Relatively Rigid Structure: Unlike the flexible hydrocarbon chains of fatty acids, the fused ring system of steroids provides a rigid, planar, or semi-planar structure, which is essential for their function.
- Amphipathic Nature (e.g., Cholesterol): While steroids are largely hydrophobic, many possess a polar hydroxyl (
-OH) group atC-3. This single polar group confers a weak amphipathic character.
Cholesterol
Cholesterol is the most well-known and biologically significant steroid in animal cells. It is exclusively found in animals and is absent from plants and bacteria.
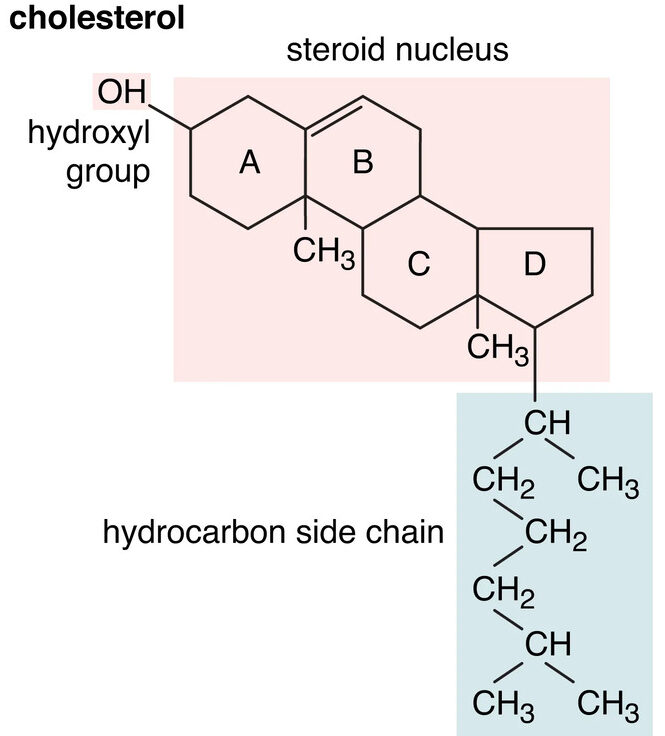
Structure:
Cholesterol possesses the characteristic four-ring steroid nucleus, along with:
- A hydroxyl group (
-OH) at C-3: This makes cholesterol a sterol (an alcohol derivative of a steroid). - A branched hydrocarbon chain attached to C-17.
- A double bond between C-5 and C-6 in ring B.
Key Functions of Cholesterol:
a. Component of Cell Membranes:
Cholesterol is a crucial component of animal cell membranes, where it is embedded within the lipid bilayer alongside phospholipids.
- Modulation of Membrane Fluidity: It acts as a "fluidity buffer":
- At higher temperatures: It decreases membrane fluidity, preventing the membrane from becoming too "liquid" or leaky.
- At lower temperatures: It increases membrane fluidity, stopping the membrane from solidifying.
- Membrane Stability: It enhances the mechanical stability and tensile strength of the membrane.
- Decreases Permeability: It reduces the permeability of the membrane to small, water-soluble molecules and ions.
b. Precursor for Other Steroids:
Cholesterol is the biochemical precursor for the synthesis of all other steroids in the body, including:
- Steroid Hormones: Signaling molecules with diverse regulatory roles.
- Bile Acids: Essential for the digestion and absorption of dietary fats.
- Vitamin D: A precursor molecule (7-dehydrocholesterol) in the skin is converted to Vitamin D₃ (cholecalciferol) upon exposure to ultraviolet (UV) light.
c. Transport in the Blood:
Being largely hydrophobic, cholesterol is transported in complexes called lipoproteins (e.g., Low-Density Lipoproteins [LDL], High-Density Lipoproteins [HDL]), which solubilize lipids for circulation in the blood.
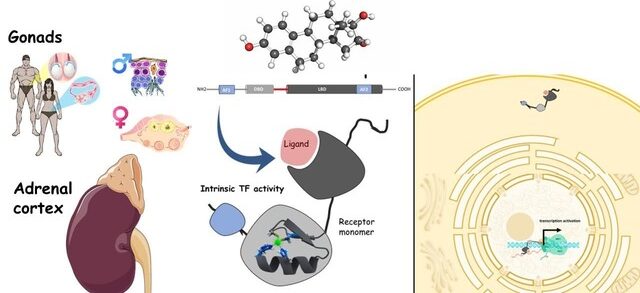
Steroid Hormones
Steroid hormones are a class of signaling molecules derived from cholesterol that play critical roles in regulating a wide range of physiological processes. They exert their effects by binding to specific intracellular receptors, subsequently modulating gene expression.
a. Glucocorticoids
(e.g., Cortisol)
Regulate metabolism, suppress inflammation, and manage the body's stress response. Produced in the adrenal cortex.
b. Mineralocorticoids
(e.g., Aldosterone)
Regulate salt/water balance and blood pressure by acting on the kidneys. Produced in the adrenal cortex.
c. Androgens
(e.g., Testosterone)
Male sex hormones promoting male secondary characteristics and muscle growth. Produced mainly in the testes.
d. Estrogens
(e.g., Estradiol)
Female sex hormones promoting female secondary characteristics and regulating the menstrual cycle. Produced mainly in the ovaries.
e. Progestogens
(e.g., Progesterone)
Involved in the menstrual cycle, maintenance of pregnancy, and embryogenesis. Produced in the ovaries, placenta, and adrenal cortex.
Mechanism of Action: Steroid hormones are lipid-soluble, allowing them to pass through the cell membrane and bind to specific intracellular receptors in the cytoplasm or nucleus. The hormone-receptor complex then binds to DNA, altering the transcription of target genes and changing cellular function.
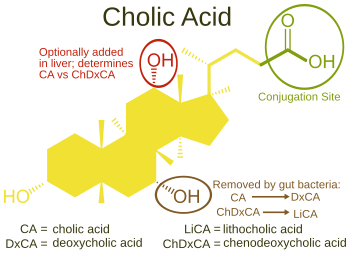
Bile Acids
- Synthesis: Bile acids are synthesized in the liver from cholesterol.
- Function: They act as powerful detergents (emulsifying agents) in the small intestine. Their primary role is to break down large dietary fat globules into smaller, more manageable fat droplets (micelles). This process, known as emulsification, significantly increases the surface area of the fats, making them more accessible for enzymatic digestion by lipases. Bile acids are also essential for the absorption of fat-soluble vitamins (A, D, E, K).
- Examples: Cholic acid and chenodeoxycholic acid are two prominent bile acids.
Other Important Lipids
This category includes diverse lipid classes that play vital structural, protective, and regulatory roles.
Waxes: Protection and Water Repellency
Waxes are simple lipids that serve primarily as protective coatings and effective water barriers in nature.
- Structure: Waxes are esters formed from the reaction of a long-chain fatty acid (14-36 carbons) and a long-chain alcohol (16-30 carbons).
- Properties:
- Extremely Hydrophobic and Water-Insoluble: Due to their very long hydrocarbon chains, waxes are exceptionally water-repellent.
- High Melting Point: They are solid at physiological temperatures, contributing to their structural integrity.
- Chemically Very Stable: Waxes are resistant to degradation, making them durable protective layers.
- Biological Functions:
- Water Repellency: Waxes form protective, water-impermeable coatings on plant leaves (cuticle), insect exoskeletons, and animal fur/feathers.
- Structural: Beeswax is secreted by worker bees to construct the robust honeycomb structures.
- Lubrication and Protection: Earwax (cerumen) in humans helps lubricate and protect the ear canal.
- Examples: Beeswax, Carnauba wax, Lanolin (wool wax).
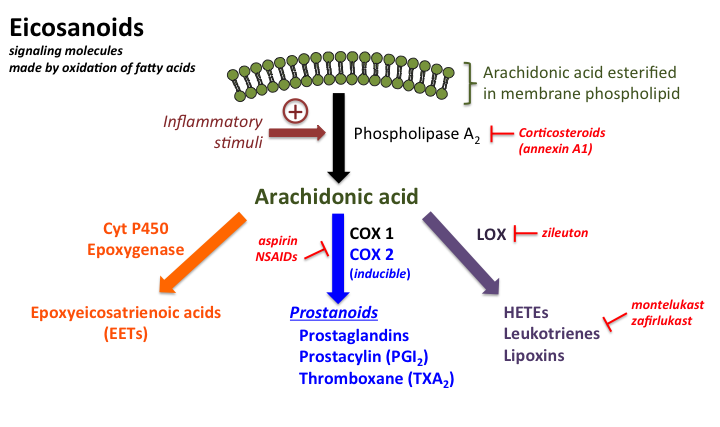
Eicosanoids
Eicosanoids are a class of incredibly potent, short-lived signaling molecules derived from 20-carbon polyunsaturated fatty acids (PUFAs).
They act as local hormones, functioning primarily in a paracrine (affecting nearby cells) and autocrine (affecting the cell that produced them) manner.
They do not circulate widely in the bloodstream like classical endocrine hormones. Their involvement spans a wide array of physiological and pathological processes, particularly those related to inflammation, immune responses, and vascular dynamics.
Precursor:
- The primary and most significant precursor for eicosanoid synthesis is arachidonic acid (AA) (
C20:4, an omega-6 fatty acid). Arachidonic acid is a component of cell membrane phospholipids and is released by the action of phospholipase A₂ (PLA₂) in response to various stimuli (e.g., tissue injury, inflammatory signals). - Other 20-carbon PUFAs, such as eicosapentaenoic acid (EPA) (
C20:5, an omega-3 fatty acid) and dihomo-γ-linolenic acid (C20:3), can also serve as precursors. Eicosanoids derived from EPA (e.g., those in the "3-series" or "5-series") are often less potent or have differing biological effects compared to those derived from arachidonic acid, frequently leading to less inflammatory or anti-aggregatory responses.
Major Classes of Eicosanoids:
The eicosanoid family is diverse, but its most prominent members include:
- Prostaglandins (PGs): Involved in inflammation, pain, fever, blood pressure regulation, blood clotting, and smooth muscle contraction.
- Thromboxanes (TXs): Primarily involved in platelet aggregation and vasoconstriction.
- Leukotrienes (LTs): Mediate allergic and inflammatory responses, particularly in the airways.
Synthesis Pathways:
The enzymatic pathways responsible for eicosanoid synthesis are distinct and targeted by various pharmacological agents:
- Cyclooxygenase (COX) Pathway: This pathway, catalyzed by the cyclooxygenase enzymes (
COX-1andCOX-2), leads to the synthesis of prostaglandins and thromboxanes.COX-1is constitutively expressed and involved in maintaining physiological functions (e.g., gastric mucosal protection, renal blood flow, platelet aggregation).COX-2is inducible, expressed primarily in response to inflammatory stimuli, and is the main enzyme responsible for prostaglandin synthesis in inflammation and pain.- This pathway is the primary target for NSAIDs (non-steroidal anti-inflammatory drugs) like aspirin and ibuprofen, which inhibit COX enzymes to reduce pain, fever, and inflammation.
- Lipoxygenase (LOX) Pathway: This pathway, catalyzed by lipoxygenase enzymes (e.g., 5-LOX), leads to the synthesis of leukotrienes.
- This pathway is targeted by some asthma medications (e.g., leukotriene receptor antagonists or 5-LOX inhibitors) to reduce bronchoconstriction and inflammation in the airways.
Biological Roles:
The roles of eicosanoids include:
- Inflammation and immune response: Modulating redness, swelling, pain, and immune cell recruitment.
- Regulation of blood pressure and blood clotting: Influencing vasoconstriction/vasodilation and platelet aggregation.
- Pain and fever induction: Acting on neural pathways.
- Reproductive processes: Such as uterine contractions during childbirth and menstruation.
- Gastric acid secretion: Influencing protective mechanisms in the stomach.
- Bronchial smooth muscle contraction/relaxation: Crucial in respiratory physiology and pathophysiology (e.g., asthma).
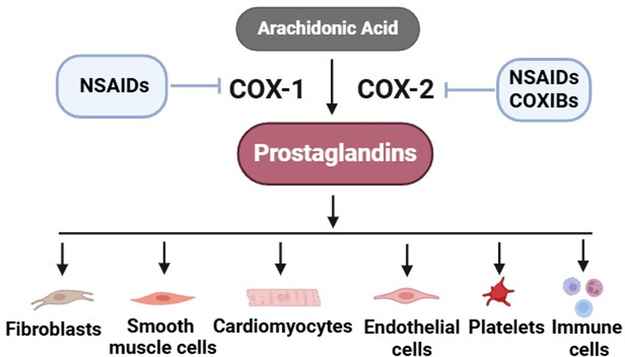
Prostaglandins (PGs)
Structure: Prostaglandins are characterized by a 20-carbon fatty acid skeleton containing a five-membered ring. The specific type (e.g., PGE, PGF) is determined by substituents on this ring. The "2-series" prostaglandins (e.g., PGE₂, PGF₂α, PGI₂) are derived from arachidonic acid.
Synthesis: Prostaglandins are synthesized from arachidonic acid via the Cyclooxygenase (COX) pathway.
Receptors: They exert their effects by binding to specific G protein-coupled receptors (GPCRs) on target cells, which elicits various intracellular signaling cascades.
Biological Functions: Prostaglandins are involved in a vast array of processes:
- Inflammation:
PGE₂is a key mediator, causing vasodilation (redness), increased vascular permeability (swelling), and sensitizing nerves to pain. - Pain: Sensitize nociceptors (pain receptors) to other pain-producing substances.
- Fever:
PGE₂acts on the hypothalamus to increase body temperature. - Reproduction:
PGF₂αplays a role in uterine contractions during labor and menstruation. - Gastrointestinal Protection:
PGE₂andPGI₂help protect the gastric mucosa by increasing mucus and bicarbonate secretion. - Renal Function: Regulate renal blood flow and electrolyte balance.
Pharmacological Significance: NSAIDs (aspirin, ibuprofen) exert their therapeutic effects by inhibiting COX enzymes, thereby reducing prostaglandin synthesis. Prostaglandin analogs are also used clinically to induce labor or treat glaucoma.
Thromboxanes (TXs)
Structure: Thromboxanes are characterized by a six-membered oxygen-containing ring. The most prominent is Thromboxane A₂ (TXA₂), which is highly unstable with a very short half-life (around 30 seconds).
Synthesis: Also synthesized via the COX pathway. The intermediate PGH₂ is converted into thromboxanes by the enzyme thromboxane synthase, which is abundant in platelets.
Biological Functions: Thromboxanes have critical roles in hemostasis:
- Platelet Aggregation:
TXA₂is an extremely potent inducer of platelet aggregation, promoting the formation of a primary hemostatic plug at the site of injury. - Vasoconstriction:
TXA₂causes potent vasoconstriction, narrowing blood vessels to reduce blood flow to an injured area. - Balance with Prostacyclin: The pro-clotting effects of
TXA₂are tightly counterbalanced by Prostacyclin (PGI₂), a vasodilator and inhibitor of platelet aggregation.
Pharmacological Significance: Low-dose aspirin irreversibly inhibits COX-1 in platelets, significantly reducing TXA₂ production for the platelet's lifetime. This antiplatelet effect is used to reduce the risk of thrombotic events like heart attack and stroke.
Leukotrienes (LTs)
Structure: Leukotrienes are linear 20-carbon derivatives (no ring structure) characterized by three conjugated double bonds. Cysteinyl Leukotrienes (cys-LTs), which include LTC₄, LTD₄, and LTE₄, are particularly potent bronchoconstrictors.
Synthesis: Synthesized from arachidonic acid via the Lipoxygenase (LOX) pathway, initiated by the 5-lipoxygenase (5-LOX) enzyme.
Biological Functions: Leukotrienes are powerful mediators of inflammation and allergic reactions:
- Bronchoconstriction: The cysteinyl leukotrienes are extremely potent bronchoconstrictors, causing airway smooth muscle to contract. This is a central feature of asthma.
- Increased Vascular Permeability: They increase the permeability of capillaries, leading to plasma leakage and edema (swelling).
- Chemotaxis and Immune Cell Recruitment:
LTB₄is a potent chemoattractant, actively recruiting neutrophils and other inflammatory cells to sites of infection or injury.
Pharmacological Significance: Given their role in respiratory and allergic diseases, leukotrienes are significant drug targets. Leukotriene Receptor Antagonists (LTRAs) like montelukast block the CysLT1 receptor to prevent bronchoconstriction. 5-Lipoxygenase Inhibitors like zileuton block leukotriene synthesis. Both are used to manage asthma.
Lipid-soluble Vitamins
Vitamins are organic compounds required in small amounts for normal metabolism but cannot be synthesized by the body in sufficient quantities. The lipid-soluble vitamins are absorbed with dietary fats and stored in lipid reserves.
Vitamin A (Retinoids)
Functions: Crucial for vision (retinal is a component of rhodopsin), essential for cell growth and differentiation (especially of epithelial tissues), supports immune function, and plays a role in reproduction.
Precursor: Beta-carotene (a carotenoid) is the most prominent provitamin A.
Deficiency: Can lead to night blindness, dry eyes (xerophthalmia), impaired immune function, and hyperkeratosis.
Vitamin D (Calciferols)
Functions: Primarily involved in the regulation of calcium and phosphate metabolism, which is essential for bone mineralization and maintaining healthy bone structure. It also plays roles in immune function and cell growth.
Synthesis: D₃ (cholecalciferol) is synthesized in the skin from a cholesterol precursor upon exposure to UV light. D₂ (ergocalciferol) is from plant sources.
Deficiency: Results in rickets in children (soft, deformed bones) and osteomalacia in adults (softening of bones).
Vitamin E (Tocopherols)
Functions: Serves as the major lipid-soluble antioxidant, primarily protecting cell membranes and other lipid-rich structures from oxidative damage caused by reactive oxygen species (free radicals). It helps maintain the integrity of cell membranes.
Deficiency: Can lead to neurological symptoms (e.g., ataxia, peripheral neuropathy) due to oxidative damage, and hemolytic anemia.
Vitamin K (Quinones)
Functions: Essential for blood clotting by participating in the synthesis of prothrombin and other clotting factors in the liver. It is also involved in bone metabolism.
Forms: Phylloquinone (K₁) is from plants; Menaquinones (K₂) are synthesized by gut bacteria.
Deficiency: Leads to impaired blood clotting, resulting in an increased risk of excessive bleeding and hemorrhage.
Carotenoids: Pigments and Antioxidants
Carotenoids are a diverse group of pigments synthesized by plants, algae, and photosynthetic bacteria. They are often responsible for the yellow, orange, and red colors of fruits, vegetables, and flowers.
- Structure: Long, conjugated polyene chains (many alternating single and double bonds), making them highly colored. They are typically
C₄₀compounds. - Properties: Highly hydrophobic.
- Biological Functions:
- Photosynthesis (in plants): Accessory pigments that absorb light energy and protect from photo-oxidative damage.
- Antioxidants: Many carotenoids (e.g., beta-carotene, lycopene) are powerful antioxidants in both plants and animals, scavenging free radicals.
- Precursors to Vitamin A: Beta-carotene is the most prominent provitamin A carotenoid; it can be cleaved to form two molecules of retinol (Vitamin A).
- Examples: Beta-carotene (carrots), Lycopene (tomatoes), Lutein and Zeaxanthin (leafy greens, important for eye health).
Prostaglandins
I. General Characteristics of Prostaglandins
- Discovery: Prostaglandins were first discovered in the 1930s by Ulf von Euler.
- Synthesis Location: They are synthesized in virtually every cell in the body, indicating their widespread biological importance.
- Molecular Structure:
- They are unsaturated 20-carbon molecules.
- A defining structural feature is that they contain a 5-member ring.
- Mode of Action:
- They work right within the cells where they are produced.
- They function as local hormones (autocrine or paracrine signaling), meaning their effects are exerted on the cells that produce them or on nearby cells.
- Stability and Storage:
- Prostaglandins have an extremely short half-life.
- They are not stored in cells; instead, they are synthesized on demand as needed.
- Biological Impact: They possess important physiological and pharmacological activities.
II. Examples of Prostaglandin Structures
PGE₂, PGF₂α, PGI₂, TXA₂ (Thromboxane A₂), LTA₄ (Leukotriene A₄)
III. Functions of Prostaglandins
- Inflammation and Pain Response: Prostaglandins are key mediators in the activation of the inflammatory response, leading to the production of pain and fever. (e.g., NSAIDs like aspirin work by inhibiting prostaglandin synthesis).
- Blood Clotting Regulation:
- Thromboxanes (e.g.,
TXA₂): These are closely related molecules that stimulate constriction of blood vessels and clotting of platelets. - Prostacyclin (
PGI₂): Conversely,PGI₂acts to inhibit clotting and dilates blood vessels. This delicate balance is vital for maintaining proper blood fluidity.
- Thromboxanes (e.g.,
- Reproductive System Involvement: Certain prostaglandins, notably
PGE₂, are involved in the induction of labor by stimulating uterine contractions. - Involvement in Other Organs and Systems:
- Kidneys: They help to regulate salt and fluid balance and increase blood flow to the kidneys.
- Gastrointestinal (GI) Tract: They increase the secretion of protective mucus and inhibit acid synthesis, shielding the stomach lining.
- Respiratory System: Related molecules, such as leukotrienes, play a role in promoting the constriction of bronchi, a key feature of conditions like asthma.
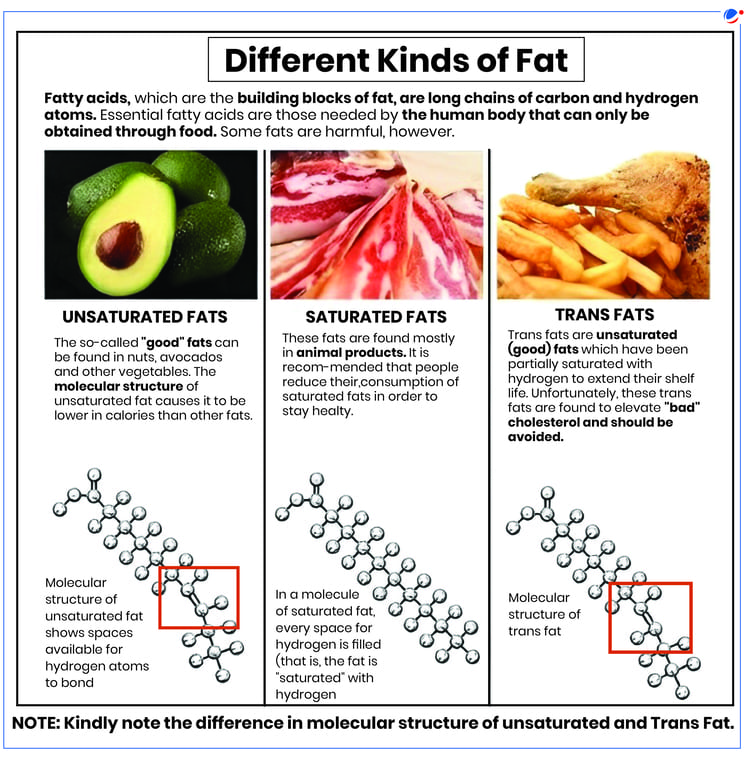
Trans Fats, Hydrogenation, and Digestion
Origin of Trans Fats: Trans fats primarily result from a process known as partial hydrogenation.
A. Full Hydrogenation:
- Process Goal: To eliminate all double bonds within unsaturated fatty acids.
- Mechanism: Hydrogen atoms are added to cis-fats (unsaturated fatty acids with cis double bonds).
- Example Illustrated: If a triglyceride contains, for instance, two double bonds, a total of 4 hydrogen atoms would be added (2 for each double bond).
- Outcome: This chemical modification effectively converts unsaturated fatty acids with cis double bonds into saturated fatty acids. The chains become fully "saturated" with hydrogen and lose their double bonds.
B. Partial Hydrogenation:
- Process Goal: Involves adding hydrogen atoms to most, but not all, of the double bonds in unsaturated fatty acids. This is a controlled, incomplete hydrogenation.
- Example Illustrated: Instead of adding 4 hydrogens as in the full hydrogenation example above, perhaps only 2 hydrogens are added to a triglyceride with two double bonds.
- Mechanism and Outcome:
- When this partial addition of hydrogen occurs, some existing double bonds are indeed converted into single bonds.
- However, during the process, some remaining double bonds can reform, but with a crucial change in their geometry: they adopt a trans configuration.
- Result: The final product is still an unsaturated fat (because not all double bonds were eliminated), but it contains trans double bonds.
Natural Occurrence:
- This process can occur naturally within the digestive tracts of certain animals, such as cows and pigs.
- Therefore, trans fats can be found naturally in small quantities in meat and dairy products.
Industrial Creation:
- Trans fats are also deliberately created through the partial hydrogenation of liquid oils.
- Purpose of Industrial Process: This process is used to alter the physical properties of oils, specifically to make them solid or semi-solid at room temperature (e.g., in margarines, shortenings), which provides desirable texture and shelf stability for food manufacturers.
Health Implications and Regulatory Action:
- Health Concern: Trans fats have been strongly associated with an increased risk of coronary heart disease.
- Response: Due to these significant health concerns, partially hydrogenated oils have been largely removed from foods in many regions, including North America and Europe.
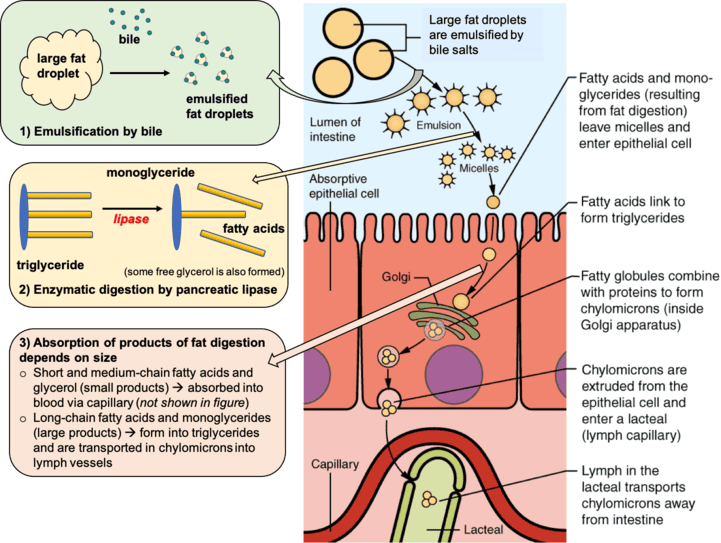
Digestion and Absorption of Dietary Fats
It's important to recognize that all foods, even those perceived as "low-fat" or "high-fat," are composed of a blend of different fatty acids, not just one type.
Initial Challenge: Hydrophobicity:
- Problem: Triglycerides are inherently hydrophobic (water-fearing).
- Consequence: When ingested, they tend to aggregate and form large globules of fat (analogous to oil floating on water). This large size makes them difficult for water-soluble digestive enzymes to access efficiently.
Steps of Digestion and Absorption:
- Emulsification (Breaking Down Large Fat Globules):
- Problem Statement: Directly working on the surface of large fat globules is an inefficient process for digestive enzymes.
- Solution Provider: Bile salts are critical for this step. They are synthesized and secreted by the liver.
- Action: Bile salts act as emulsifiers, breaking the large fat droplets into much smaller droplets.
- Effect: This emulsification dramatically increases the surface area available for digestive enzymes (lipases) to work on, significantly speeding up digestion.
- Enzymatic Breakdown (Hydrolysis of Triglycerides):
- Enzymes Involved: A class of enzymes called lipases are responsible for breaking down fats. These are found in saliva (lingual lipase), the stomach (gastric lipase), and are secreted by the pancreas (pancreatic lipase).
- Action: Lipases hydrolyze (break down using water) triglycerides.
- Resulting Products: Triglycerides are broken down into free fatty acids and monoglycerides.
- Micelle Formation (Packaging for Transport):
- Assembly: After breakdown, the monoglycerides and free fatty acids, along with other lipid-soluble substances, spontaneously self-assemble into mixed micelles.
- Micelle Structure: These are small, spherical structures with a hydrophobic interior and a hydrophilic ("water-loving") exterior, allowing the micelle to be water-soluble.
- Function: Micelles enable the transport of the lipid digestion products through the watery environment of the intestinal lumen.
- Absorption into Enterocytes (Intestinal Cells):
- Journey: The micelles "glide" through the intestinal lumen until they reach the surface of the enterocytes (the absorptive cells lining the intestinal wall).
- Release and Diffusion: When they reach the enterocytes, the micelles release their cargo (fatty acids and monoglycerides), which then diffuse into the enterocyte across its membrane.
- Re-esterification and Chylomicron Formation (Inside the Enterocyte):
- Reassembly: Once inside the enterocyte, the free fatty acids and monoglycerides are reassembled back into triglycerides.
- Packaging: These newly reformed triglycerides, along with cholesterol and fat-soluble vitamins (A, D, E, K), are then packaged into a much larger lipoprotein structure called a chylomicron.
- Chylomicron Structure: It has an outer membrane of phospholipids and proteins (apolipoproteins) and a hydrophobic core containing the triglycerides and other lipids.
- Transport via the Lymphatic System (Bypassing the Liver Initially):
- Exit from Enterocyte: The chylomicrons are too large to directly enter the bloodstream capillaries.
- Entry to Lacteal: Instead, they enter a specialized lymphatic capillary called a lacteal.
- Lymphatic Flow: The chylomicron travels through the lymphatic vessels.
- Entry to Bloodstream: The lymphatic system eventually drains into the circulatory system at the thoracic duct.
- Significance: This pathway means that dietary fats, packaged as chylomicrons, initially bypass direct processing by the liver.
- Delivery to Peripheral Tissues:
- Circulation: Once in the bloodstream, chylomicrons circulate throughout the body.
- Release of Contents: Enzymes (primarily lipoprotein lipase) on capillary walls act on the chylomicrons, causing them to release fatty acids and monoglycerides.
- Utilization: These lipids are taken up by muscle cells for energy or by adipose tissue for storage.
- Chylomicron Remnant Clearance:
- Shrinkage: After delivering most of their triglyceride cargo, the chylomicrons become smaller, now referred to as chylomicron remnants.
- Liver Uptake: These remnants are eventually recognized and engulfed by the liver for further processing.