Bioenergetics : Thermodynamics & ATP
Bioenergetics:
The Engine of Life – How Organisms Manage Energy
Let's shift our focus now to the fundamental concept of Bioenergetics. The term itself is quite descriptive:
- "Bio" means life.
- "Energetics" means the study of energy.
Therefore, Bioenergetics is simply the study of how living organisms manage energy. It's the exploration of the transfer and utilization of energy in biological systems. It delves into the intricate mechanisms that allow life to exist and thrive, from the smallest bacteria to the largest whales.
This critical field encompasses several key aspects:
- How organisms obtain energy: This involves understanding the initial sources of energy.
- How organisms transform this energy from one form to another: Energy needs to be converted into a usable "currency".
- How organisms use this energy to do all the things necessary for life: Fueling every biological process.
At its core, Energy means the capacity or ability to do work.
In biology, "work" means anything that requires effort or causes a change. It's a much broader concept, encompassing all the dynamic processes that sustain life. Just like machines require energy to do work, all living organisms need a constant supply of energy to function.
Examples of "Work" in Biological Systems Requiring Energy:
Gross Motor Movement (Physical Work)
Just as a car needs petrol, our muscles need energy to contract and allow us to walk, run, or lift objects. Our heart muscle continuously contracts to pump blood, and our diaphragm contracts to allow us to breathe.
Growth and Development (Synthetic Work)
A toddler growing into an adult requires a massive amount of energy to synthesize new cells, tissues, and complex molecules like proteins and DNA. This is "building work." The process of reproduction also demands significant energy input.
Maintaining Homeostasis (Maintenance Work)
Even when resting, our body is still doing immense "work"! This basal metabolic activity includes our heart beating, lungs breathing, brain activity, and cells constantly repairing themselves and actively transporting ions across membranes.
Where does this Energy Come From? The Sun
For planet Earth, the main, original, and most abundant source of energy is sunlight. However, most living things, including humans, can't directly use sunlight. It's a fascinating journey through the food chain.
The Journey of Sunlight Energy to You:
-
Plants (Producers – The Solar Collectors):
Plants capture light energy from the sun through a process called photosynthesis. They use simple molecules like CO₂ and water to convert this light energy into glucose, a form of stored chemical energy. -
Animals (Consumers – The Energy Transfer Agents):
When you eat a plant product, you are directly consuming the glucose the plant made. When you eat an animal product, that animal likely consumed plants, so you are indirectly getting the energy that originally came from the sun.
The Goal: Energy-Rich Molecules for Your Cells
Regardless of what you eat, your body obtains these energy-rich molecules (carbohydrates, fats, proteins). Your cells then break them down through metabolic pathways to release the stored chemical energy. This released energy is then used to synthesize a special energy currency molecule called ATP (Adenosine Triphosphate), which is the direct fuel for almost all cellular work.
Clinical Relevance for Nursing Students
Understanding bioenergetics is foundational to many aspects of nursing:
- Nutrition and Energy Intake: Nurses assess patients' nutritional status and energy needs for processes like wound healing and recovery. Malnutrition directly impacts a patient's ability to heal.
- Metabolic Disorders: Diseases like diabetes mellitus are direct examples of impaired bioenergetics—the body's inability to properly utilize glucose for energy.
- Exercise Physiology: The energy demands of physical activity are direct applications of bioenergetics.
- Pharmacology: Many drugs affect metabolic pathways, altering how cells produce or use energy.
- Patient Education: Explaining the importance of a balanced diet can empower patients to manage their health effectively.
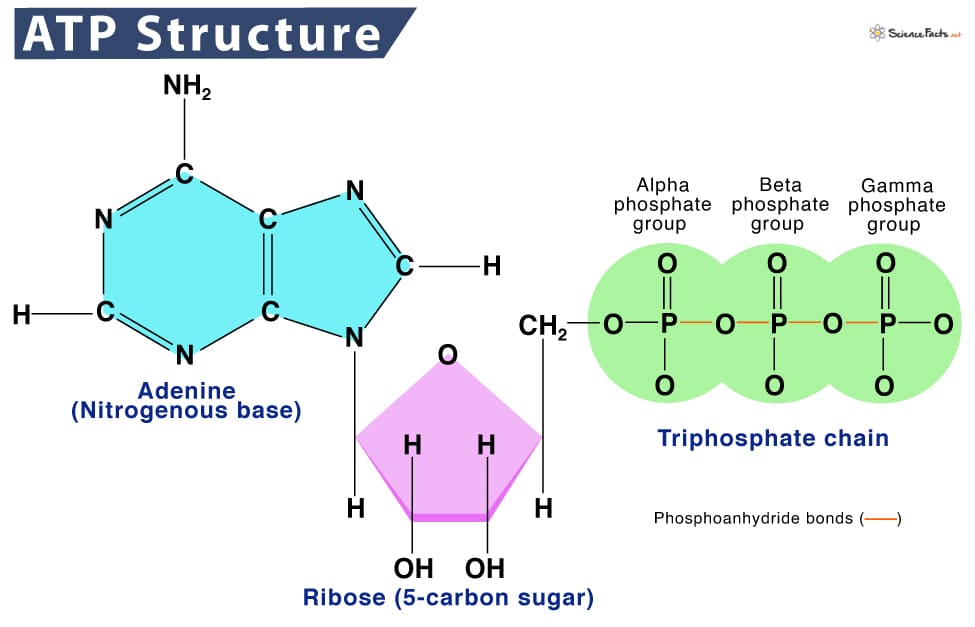
ATP: The Body's Universal Energy Currency
Once the body gets energy from food, it doesn't directly use these complex food molecules to power every single tiny process. Instead, the body converts the chemical energy stored in these food molecules into a much more manageable and readily available form: a special molecule called ATP.
ATP: Adenosine Triphosphate
Why is ATP called the "Energy Currency"? Think of it like money. You don't get paid in raw materials; you get paid in money, which you can use to buy whatever you need. Similarly, your body converts energy from diverse food sources into ATP (the "money"). Then, it uses ATP to "pay for" all its energy-requiring processes.
ATP is the direct, usable form of energy for almost all cellular activities.
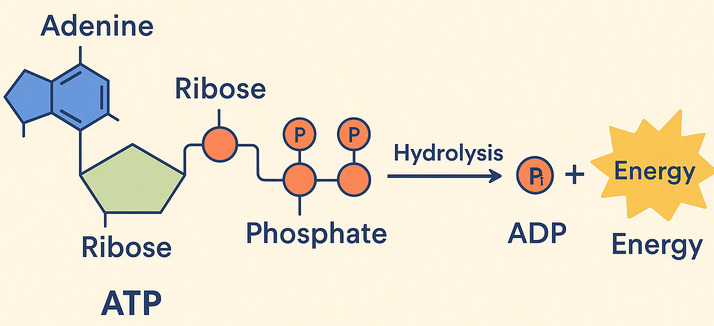
How does ATP store and release energy?
The key lies in the "high-energy" chemical bonds connecting its three phosphate groups. When your cells need energy, they break off one of the phosphate groups from ATP. This breaking of the bond releases a significant amount of free energy that the cell can immediately use.
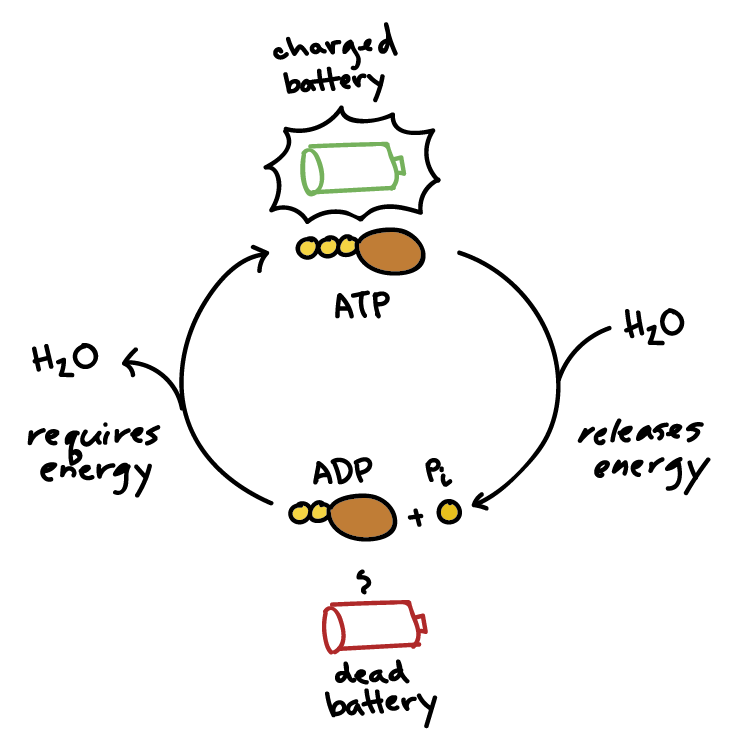
ATP → ADP + Pᵢ + Energy
This reaction is reversible. When your body has excess energy, it can use it to reattach the phosphate group to ADP, converting it back into ATP, thus "recharging the battery."
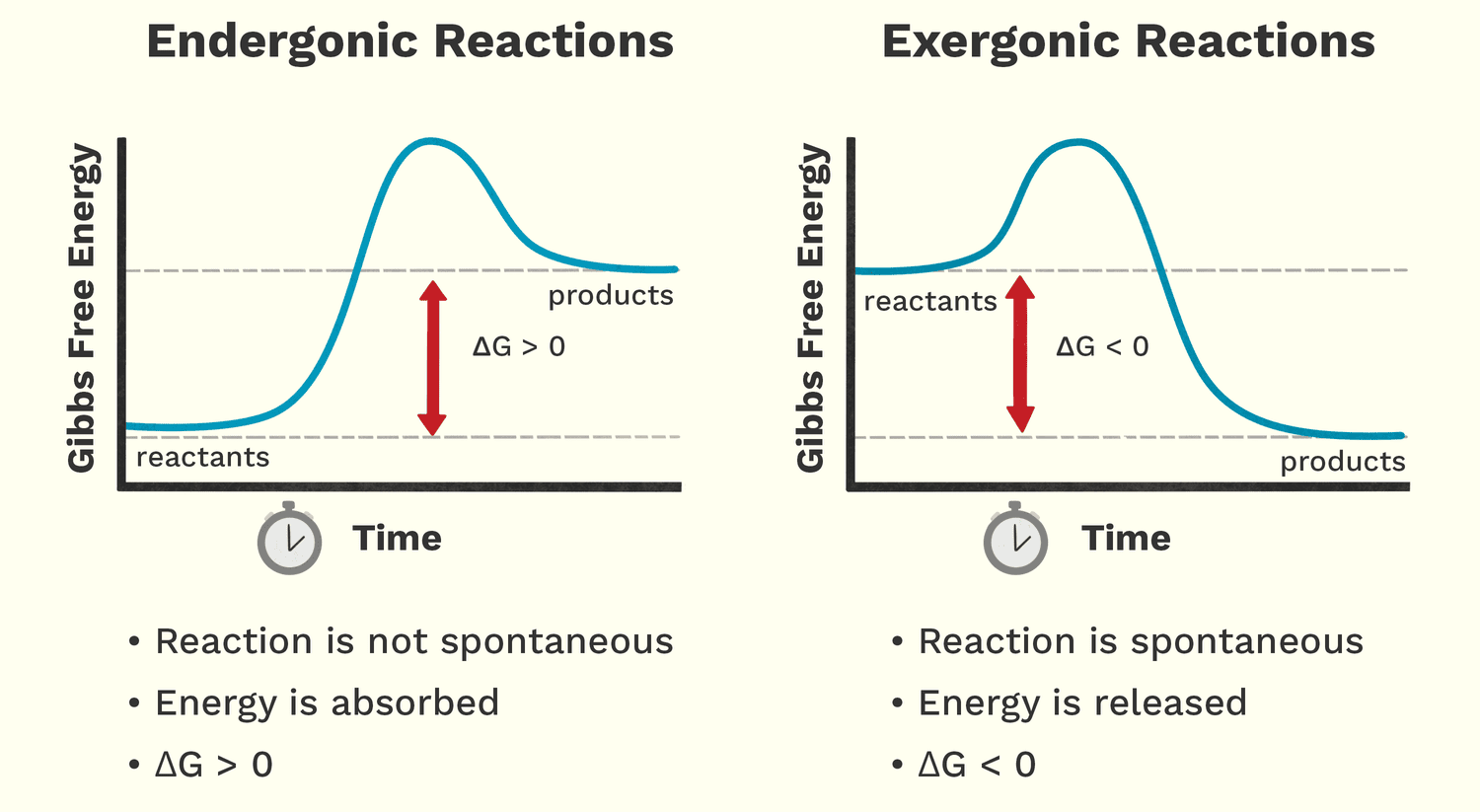
Free Energy (Exergonic vs. Endergonic Reactions)
Free energy (Gibbs Free Energy, G) is the amount of energy available to do work within a system. It helps us predict whether a chemical reaction will happen spontaneously (release energy) or require an input of energy.
Analogy: A person moving down a hill is a spontaneous process that releases energy. A person lifting a weight up a hill is a non-spontaneous process that requires energy.
Exergonic Reactions: Energy-Releasing
These reactions release free energy and can happen spontaneously. The change in free energy (ΔG) is negative (ΔG < 0).
Biological Examples:
- Cellular Respiration: The breakdown of glucose into CO₂ and water releases a lot of free energy used to make ATP.
- ATP Hydrolysis: The breakdown of ATP into ADP + Pᵢ is a classic exergonic reaction that releases usable energy for the cell.
Endergonic Reactions: Energy-Requiring
These reactions require an input of free energy and are non-spontaneous. The change in free energy (ΔG) is positive (ΔG > 0).
Biological Examples:
- Protein Synthesis: Building a complex protein from individual amino acids requires significant energy.
- Muscle Contraction: The process of muscle fibers shortening is an endergonic process powered by ATP.
- Active Transport: Moving substances across a cell membrane against their concentration gradient always requires energy.
The Critical Relationship: Energy Coupling
Life thrives by ingeniously linking these two types of reactions together. Cells use the energy released from an exergonic reaction (like ATP breaking down) to drive an endergonic reaction that needs energy. This is called energy coupling. ATP is the perfect intermediate, acting as the bridge that carries energy from energy-releasing pathways to energy-requiring processes.
Clinical Relevance for Nursing Students
- Cellular Function and Disease: Many diseases, like heart failure, involve inefficient energy production or utilization by cells, affecting their ability to perform endergonic work.
- Medication Impact: Some drugs target enzymes involved in ATP production or use, impacting the cell's ability to do work.
- Understanding Pathophysiology: When a patient is fatigued or weak, it often points to issues with their body's ability to generate or use ATP.
- Wound Healing and Tissue Repair: These are highly endergonic processes that require a massive input of ATP. Patients with poor nutrition or impaired energy metabolism will have difficulty healing.
Thermodynamics:
The Universal Rules of Energy
The overarching scientific field that governs all energy concepts is Thermodynamics. It is a branch of science that deals with the transformation or interconversion of different forms of energy, and how that energy is utilized.
Literally, thermodynamics is about the power of heat or the movement of heat and energy. While "heat" is in the name, it encompasses all forms of energy relevant to biological systems, including light, thermal, chemical, electrical, and mechanical energy.
The Laws of Thermodynamics: Unbreakable Rules of the Universe
Thermodynamics is built upon a few fundamental principles known as the Laws of Thermodynamics. These laws are absolute and govern all energy transformations in the universe, including those happening inside the human body.
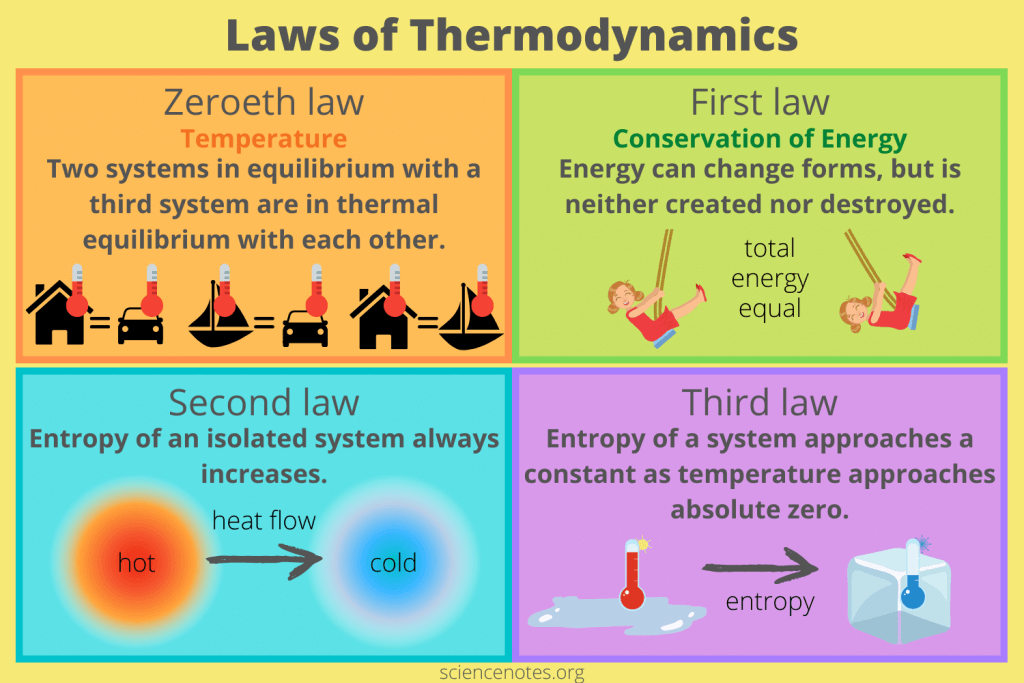
Zeroeth Law of Thermodynamics: Defining Temperature
"Two systems in equilibrium with a third system are in thermal equilibrium with each other."
Meaning: This law defines temperature and is the principle that allows a thermometer to accurately measure a patient's temperature.
Biological Implication: This law underpins the concept of body temperature and thermoregulation. Our bodies constantly strive to maintain a thermal equilibrium (homeostasis).
The First Law of Thermodynamics: Conservation of Energy
"Energy cannot be created or destroyed, only transformed from one form to another."
Meaning: The total amount of energy in the universe is constant. You can't get something for nothing.
Biological Implications: Plants don't "make" energy; they transform light energy into chemical energy. When you exercise, you convert chemical energy from food into mechanical energy and heat. Life needs a constant input of energy because organisms are continuously transforming it from external sources to fuel internal processes.
The Second Law of Thermodynamics: The Increase of Entropy
"In any isolated system, the total entropy (disorder) can only increase or remain constant."
Meaning: The universe naturally tends towards a state of greater disorder, randomness, or chaos. Things naturally fall apart; they do not spontaneously become more organized without external effort.
Biological Implications: Living organisms are incredibly complex, highly ordered structures. To maintain this order and fight against entropy, organisms must constantly consume energy. Life is a continuous battle against the Second Law. Every energy transformation results in some energy being "lost" as unusable heat, increasing the entropy of the environment.
Third Law of Thermodynamics: Absolute Zero and Order
"The entropy of a system approaches a constant minimum value as its temperature approaches absolute zero."
Meaning: As a system's temperature gets closer to absolute zero (-273.15 °C), the disorder of the system approaches a minimum. At absolute zero, a perfect crystal would theoretically have zero entropy (perfect order).
Biological Implication: This law highlights the relationship between temperature and molecular motion/disorder. Very low temperatures reduce molecular motion, which is why cryopreservation attempts to halt metabolic processes by drastically reducing temperature and entropy.
The Link to Bioenergetics: Thermodynamics as the Foundation
Bioenergetics is essentially the application of thermodynamic principles to biological systems. It helps us understand:
- How organisms obtain and transform energy (First Law).
- How they use energy to do work (First and Second Laws).
- Why they constantly need more energy to maintain life against the forces of entropy (Second Law).
Clinical Relevance for Nursing Students
These laws have direct clinical applications:
- Fever and Hypothermia: Understanding the Zeroeth Law contextualizes temperature regulation. A fever is an active, energy-intensive process, while hypothermia is a state where energy production cannot keep up with heat loss.
- Metabolic Rate: Basal Metabolic Rate (BMR) is a direct application of the First Law – how much energy a patient takes in versus how much they transform and use. This is crucial for managing nutrition and recovery.
- Cellular Degeneration and Aging: The Second Law helps explain the natural tendency for cells and tissues to break down over time. Aging is a continuous battle against increasing entropy.
- Wound Healing and Recovery: These processes are highly anabolic (building up), requiring significant energy input to create order (new tissue) and fight against entropy.
ATP: The Body's Perfect Energy Currency – A Closer Look
We've already introduced ATP as the energy currency that cells use to "pay for" their work. Now let's understand exactly how this remarkable molecule functions in this essential role.
1. The Role of "High-Energy" Bonds in ATP
ATP (Adenosine Triphosphate) is made of adenosine and three phosphate groups. The key to its power lies in the bonds between these phosphate groups, often called "high-energy phosphate bonds."
The term "high-energy" refers to the fact that when these bonds are broken, a significant amount of free energy is readily released. This is because the three negatively charged phosphate groups strongly repel each other, creating strain. Breaking the bond reduces this repulsion, and the remaining molecules (ADP and Pᵢ) settle into a more stable, lower-energy state. The difference in energy is what the cell can harness.
The Reaction: ATP Hydrolysis (Spending the Currency)
When the cell needs energy, it breaks the outermost phosphate bond in a process called hydrolysis, because a molecule of water (H₂O) is used to break the bond.
ATP + H₂O → ADP + Pᵢ + Free Energy
2. How Energy is "Coupled" to Power Reactions
This is truly the magic of ATP! It perfectly acts as the bridge between energy-releasing (exergonic) and energy-requiring (endergonic) processes.
The ATP Cycle & The "Coupling" Mechanism: Phosphorylation
Life depends on a continuous, rapid cycle of ATP breakdown and synthesis:
- Recharging (Endergonic): Energy from the breakdown of food is used to add a phosphate group back to ADP, reforming ATP.
ADP + Pᵢ + Energy (from food) → ATP + H₂O - Using (Exergonic): When the cell needs to perform an endergonic task, it "spends" an ATP molecule.
ATP + H₂O → ADP + Pᵢ + Free Energy (for work)
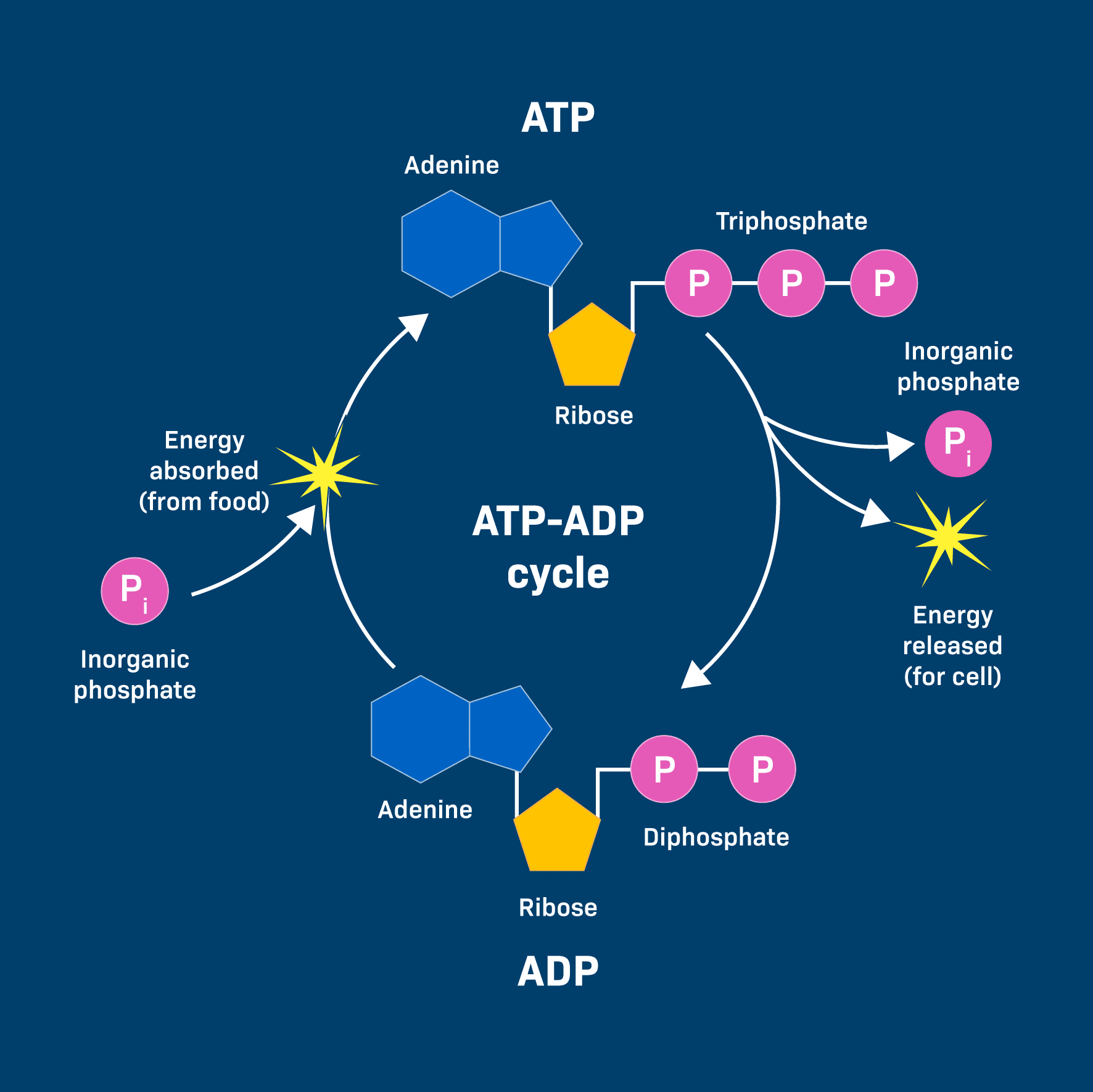
This energy is often transferred through a clever mechanism called phosphorylation. The phosphate group released from ATP is temporarily transferred to another molecule. This energizes the receiving molecule, making it more reactive and priming it to undergo its desired endergonic reaction.
Example: Muscle Contraction
An ATP molecule binds to a muscle protein (myosin). The ATP is hydrolyzed, and the phosphate (Pᵢ) temporarily attaches to the protein (phosphorylation). This causes a change in the protein's shape, leading to the physical contraction (the "work").
Why is ATP so perfect for this role?
- Manageable Energy Packet: It releases just the right amount of energy for most cellular reactions—not too much to be wasteful, and not too little to be ineffective.
- Universal Currency: Nearly all organisms and cellular processes use ATP.
- Rapid Turnover: Cells can quickly break down and resynthesize ATP, providing a constant and immediate supply of energy. A typical human adult can turn over their entire body weight in ATP every single day!
So, to summarize the continuous flow of energy that powers life:
- Sunlight → Photosynthesis (in plants) stores energy in glucose.
- Digestion breaks down glucose and other food molecules.
- Cellular Respiration (exergonic) breaks down these molecules to generate ATP from ADP.
- ATP hydrolysis (exergonic) provides bursts of free energy to power the cell's endergonic reactions (building, moving, signaling).
Clinical Relevance for Nursing Students
Understanding ATP's role is fundamental to comprehending cellular health:
- Cellular Function and Failure: When ATP production is compromised (e.g., in oxygen deprivation or metabolic poisons), cells cannot perform their essential endergonic tasks, leading to cellular damage and organ failure.
- Cardiac Function: The heart is a massive consumer of ATP. A heart attack involves a lack of oxygen, which cripples ATP production and leads to heart muscle damage.
- Pharmacology: Many drugs work by affecting ATP production or utilization pathways.
- Energy Demands of Illness: Patients recovering from surgery, trauma, or infection have significantly increased energy demands. Their bodies need to synthesize new proteins and repair tissues—all highly endergonic processes fueled by ATP.
- Diabetic Ketoacidosis (DKA): In DKA, cells cannot effectively use glucose to make ATP. They turn to fat breakdown, leading to an accumulation of ketones and acidosis, highlighting a major disruption in energy metabolism.
Understanding Enthalpy and Entropy (and the Gibbs Free Energy Equation)
We previously touched upon the Second Law of Thermodynamics, which introduced the powerful idea that things naturally tend towards disorder. This concept is called entropy, and it's a critical component of understanding where "free energy" comes from.
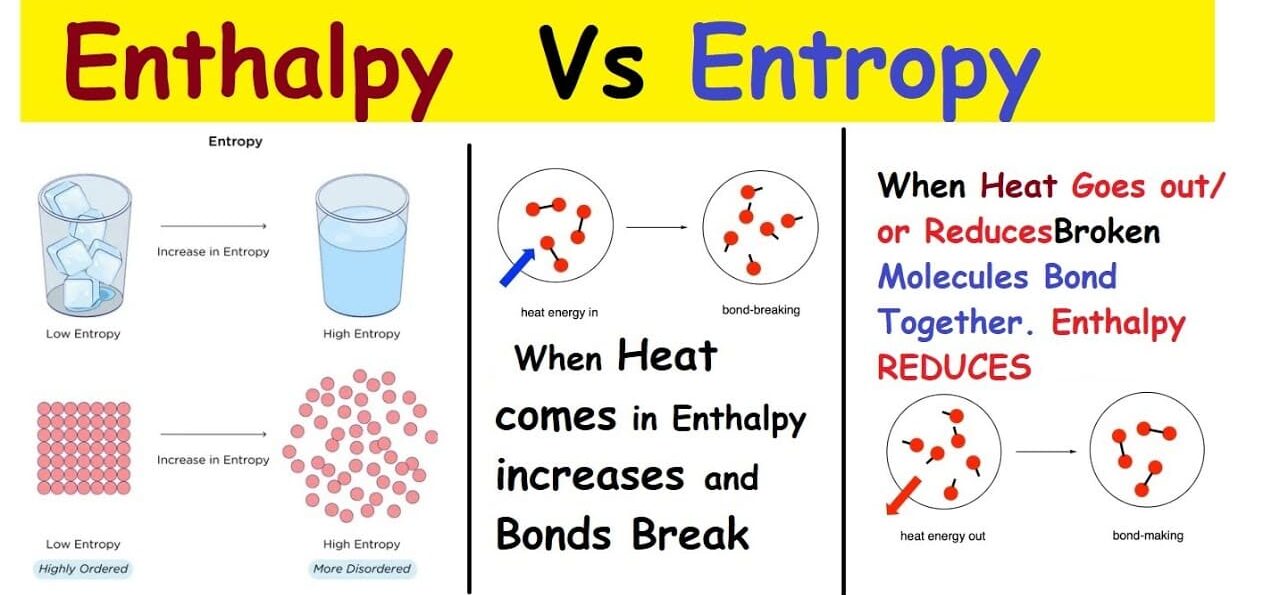
What is Entropy (ΔS)?
Entropy (S) is a fundamental thermodynamic property that serves as a quantitative measure of randomness or disorder within a system. The more ways particles can be arranged, or the more freely they can move, the higher the entropy.
Analogy: Generally, gases (high entropy, chaotic) have higher entropy than liquids (medium entropy, less ordered), which have higher entropy than solids (low entropy, ordered). Breaking large, complex molecules into smaller, simpler ones also increases entropy.
What is Enthalpy (ΔH)?
Enthalpy (H) is essentially the total heat content or the total potential energy contained within a system at constant pressure. We are most interested in the change in enthalpy (ΔH).
- Exothermic Reactions: A reaction that releases heat into the surroundings has a negative ΔH.
- Endothermic Reactions: A reaction that absorbs heat from the surroundings has a positive ΔH.
The Gibbs Free Energy Equation: ΔG = ΔH - TΔS
This powerful equation is the heart of bioenergetics because it connects these concepts to determine whether a reaction will be spontaneous (exergonic) or require energy (endergonic).
- ΔG (Change in Gibbs Free Energy): The amount of useful energy available to do work.
- A negative ΔG indicates an exergonic reaction (spontaneous, energy-releasing).
- A positive ΔG indicates an endergonic reaction (non-spontaneous, energy-requiring).
- ΔH (Change in Enthalpy): The change in total heat content. A negative ΔH (releasing heat) favors spontaneity.
- T (Temperature): The absolute temperature in Kelvin.
- ΔS (Change in Entropy): The change in disorder. A positive ΔS (increasing disorder) favors spontaneity.
Reactions are most likely to be spontaneous (exergonic) if they release heat (negative ΔH) AND increase disorder (positive ΔS).
Applying to Biological Reactions: Photosynthesis vs. Cellular Respiration
A. Photosynthesis (Endergonic)
6CO₂ + 6H₂O + Light → C₆H₁₂O₆ + 6O₂
- ΔS is negative: Simple, disordered molecules (CO₂, H₂O) are used to build a large, complex, ordered molecule (glucose). Entropy decreases.
- ΔH is positive: Energy from sunlight is absorbed to build the high-energy bonds in glucose. The reaction is endothermic.
- Overall ΔG is positive: Since both a positive ΔH and a negative ΔS make ΔG positive, photosynthesis is a highly endergonic reaction. It requires a continuous input of energy.
B. Cellular Respiration (Exergonic)
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + Energy
- ΔS is positive: A complex, ordered molecule (glucose) is broken down into simpler, more disordered molecules (CO₂, H₂O). Entropy increases.
- ΔH is negative: The energy-rich bonds in glucose are broken, releasing stored energy as ATP and heat. The reaction is exothermic.
- Overall ΔG is negative: Since both a negative ΔH and a positive ΔS make ΔG negative, cellular respiration is a highly exergonic reaction. It releases a significant amount of free energy.
Clinical Relevance for Nursing Students
- Metabolic Rate and Heat Production: The ΔH component explains why our bodies generate heat. Cellular respiration is exothermic (negative ΔH), contributing to our core body temperature.
- Nutritional Support: Building tissues (e.g., wound healing) are endergonic processes (positive ΔG). They require significant energy input from food to proceed.
- Fever Management: Temperature (T) is a critical component of the equation. A higher T can increase the rate of some reactions but can also denature proteins if it gets too high.
- Cellular Homeostasis and Disease: Maintaining cellular order (low entropy) requires constant energy expenditure. Diseases often arise when a cell's ability to generate or use ATP breaks down, leading to an increase in cellular disorder.
- Drug Action: Some medications might influence the ΔH or ΔS of metabolic reactions, altering their favorability or rate.
Phosphoryl Group Transfers:
The Core Mechanism of ATP Energy
We've talked about ATP hydrolysis as releasing energy, but how does that energy actually get used? The primary way is through phosphoryl group transfer, often referred to simply as phosphorylation.
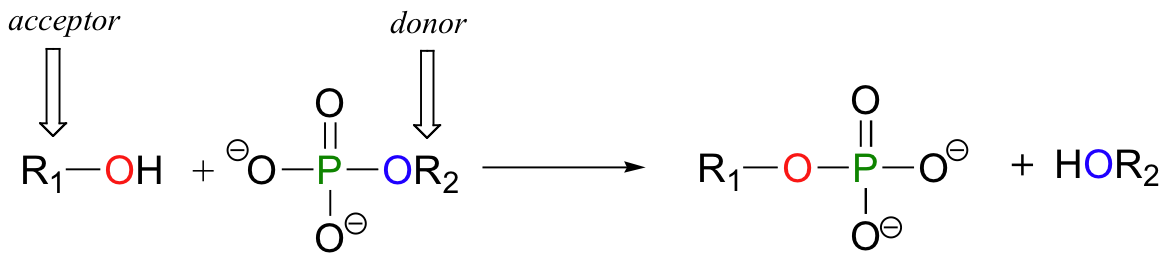
What is a Phosphoryl Group Transfer?
A phosphoryl group transfer is the movement of a phosphate group (Pᵢ) from one molecule to another. ATP is the most common donor. The enzyme-catalyzed transfer of the terminal phosphate group from ATP to a recipient molecule results in a phosphorylated recipient and ADP.
Why is this so effective for "energy coupling"?
- Raises the Free Energy of the Recipient: Adding a phosphate group "energizes" or "activates" the recipient molecule, making it less stable and more reactive.
- Makes Reactions More Favorable: The now-phosphorylated molecule is in a higher energy state, which can make a previously unfavorable (endergonic) reaction spontaneous.
- Induces Conformational Changes: The addition of a bulky, charged phosphate group can change a protein's shape, which is critical for processes like muscle contraction, active transport pumps, and signal transduction.
- Enzyme Regulation: Phosphorylation is a key mechanism for turning enzymes on or off.
Clinical Relevance for Nursing Students
- Cellular Function and Dysfunction: Nearly every cellular process relies on phosphoryl group transfers. Disruption of these pathways can have widespread and severe consequences.
- Drug Targets: Many drugs work by targeting enzymes involved in phosphorylation (e.g., kinase inhibitors used in cancer therapy).
- Metabolic Disorders: In conditions like diabetes, the body's ability to properly phosphorylate glucose is impaired.
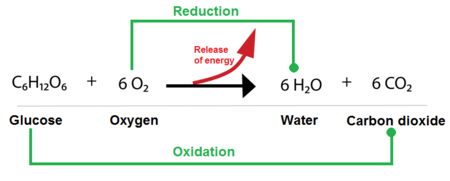
Biological Oxidation-Reduction (Redox) Reactions:
The Energy Harvest
While phosphoryl group transfers are about using energy, oxidation-reduction (redox) reactions are primarily about harvesting and transferring energy from nutrient molecules. This is how cells extract energy from food.
What are Oxidation and Reduction?
These are always coupled reactions:
- Oxidation: The loss of electrons (and often hydrogen atoms).
- Reduction: The gain of electrons (and often hydrogen atoms).
A helpful mnemonic is LEO the lion says GER! (Lose Electrons Oxidation, Gain Electrons Reduction).
In biological systems, the transfer of electrons often happens along with the transfer of protons (H⁺), so oxidation often means losing hydrogen atoms (dehydrogenation), and reduction often means gaining them (hydrogenation).
Electron Carriers: The "Couriers" of Redox Energy
Cells use specialized molecules to pick up and carry electrons. The two most important are:
- NAD⁺ (Nicotinamide Adenine Dinucleotide): Its oxidized form is NAD⁺. Its reduced form, NADH, carries 2 electrons and 1 proton.
- FAD (Flavin Adenine Dinucleotide): Its oxidized form is FAD. Its reduced form, FADH₂, carries 2 electrons and 2 protons.
The Overall Flow of Energy through Redox Reactions:
- Nutrient Oxidation: Glucose is gradually oxidized in pathways like glycolysis and the Krebs cycle.
- Electron Carrier Reduction: The released electrons are picked up by NAD⁺ and FAD, reducing them to NADH and FADH₂.
- Electron Transport Chain (ETC): NADH and FADH₂ deliver these high-energy electrons to the ETC in the mitochondria.
- Energy Release: As electrons pass down a series of protein complexes, they move from a higher to a lower energy state, releasing energy.
- ATP Synthesis (Oxidative Phosphorylation): This released energy is used to pump protons, creating a gradient that drives the synthesis of large amounts of ATP.
- Oxygen as Final Electron Acceptor: At the end of the ETC, oxygen accepts the "spent" electrons and combines with protons to form water. This is why we breathe oxygen!
Clinical Relevance for Nursing Students
- Oxygen Dependence: The critical role of oxygen as the final electron acceptor highlights why hypoxia (low oxygen) severely impairs ATP synthesis, leading to cellular damage and death.
- Metabolic Poisons: Substances like cyanide block the ETC, immediately halting ATP production, which is why they are so fatal.
- Mitochondrial Diseases: Genetic disorders affecting the ETC can severely compromise a patient's ability to produce ATP, affecting high-energy organs like the brain, muscles, and heart.
- Nutritional Deficiencies: Deficiencies in vitamins that are precursors to NAD⁺ (Niacin/B3) and FAD (Riboflavin/B2) can impair electron transport and ATP production.
- Exercise and Fatigue: During intense exercise, when oxygen supply is limited, cells switch to less efficient anaerobic metabolism, which produces far less ATP.
Biochemistry Lesson Four
Bioenergetics
Test your knowledge with these 20 questions.
Biochemistry: Bioenergetics
Question 1/20
Quiz Complete!
Here are your results, .
Your Score
18/20
90%