Biochemistry Intro : Atoms and Molecules
Phase 1: Chemistry Fundamentals
Objectives
At the end of this section, you will be able to understand:
- Atoms and Molecules:
- What are atoms? Protons, neutrons, electrons.
- The periodic table (its organization, not memorization).
- What are molecules? How do atoms join together?
- Why it's important: Biochemistry is all about molecules in living systems. You need to know what they are made of.
Without a chemistry background, Let's Review Chemistry.
Chemistry is the study of matter and the ways in which different forms of matter combine with each other.
You study chemistry because it helps you to understand the world around you.
Everything you touch or taste or smell is a chemical, and the interactions of these chemicals with each other define our universe. Chemistry forms the fundamental basis for biology and medicine.
Areas of Chemistry
The study of modern chemistry has many branches, but can be broken down into five main disciplines, or areas of study:
Physical chemistry
The study of macroscopic properties, atomic properties, and phenomena in chemical systems.
Organic chemistry
The study of chemicals containing carbon.
Inorganic chemistry
The study of chemicals that, in general, are not primarily based on carbon.
Analytical chemistry
The study of the composition of matter.
Biochemistry
The study of chemical processes that occur in living things.
Biology is the scientific study of life and living organisms, from the smallest single cells to entire ecosystems. It pays attention to the organization of life, their functions, patterns, growth, and development.
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. Biochemistry may be divided into three fields: structural biology, enzymology, and metabolism.
Carl Neuberg is considered the "father of modern biochemistry" for his work in the field, including discovering carboxylase and elucidating alcoholic fermentation.
For a beginner, let's go back in time and start from matter 😂😂
What is the matter?
Matter is anything that occupies space and has weight. Literally.
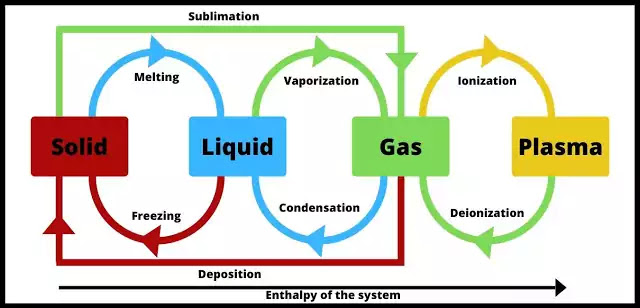
States of Matter
Matter exists in various physical forms, each characterized by distinct particle arrangements and behaviors. Understanding these states is important for comprehending physiological processes and medication properties.
1. Solid State
Particles are tightly packed in a fixed, orderly pattern, giving solids a definite shape and volume.
Examples: Bone, pharmaceutical tablets, ice.
2. Liquid State
Particles are close but can move past each other, allowing liquids to flow and take the shape of their container. They have a definite volume but an indefinite shape.
Examples: Blood, intravenous fluids, water.
3. Gaseous State
Particles are far apart and move randomly and rapidly. They have neither a definite shape nor volume and will expand to fill any container.
Examples: Oxygen, anesthetic agents, air in the lungs.
4. Plasma State
An ionized gas where some electrons have been stripped from atoms. It is the most abundant state in the universe. It has an indefinite shape and volume but can conduct electricity.
Examples: Lightning, stars.
Characteristics of matter
Objects are distinguished from each other by their physical and chemical properties.
Physical Properties
- Colour, taste and smell
- Density
- Melting point
- Boiling point
- Hardness
- Electric conductivity
- Thermal conductivity
Chemical Properties
- Reactivity
- Flammability
- Acid and Basicity
- Corrosivity
- Toxicity
- Oxidation
- Radioactivity
Physical Properties
These are characteristics that can be observed or measured without changing the identity of the substance.
A. Colour, Taste, and Smell
Used to differentiate between substances like gold vs. iron, salt vs. sugar, and perfume vs. vinegar.
B. Density
The mass per unit volume of matter. Materials with a higher density than water sink, while those with lower density float. This is why water isn't used for petrol fires and why helium balloons rise.
C. Melting Point
The temperature at which a solid changes to a liquid. Cooking pots are made of materials with high melting points.
D. Boiling Point
The temperature at which a liquid changes to a gas. The separation of petroleum oil components is based on their different boiling points.
E. Hardness
The resistance of a solid to being scratched or dented. Screwdrivers and building rods are made of extremely hard steel iron.
F. Electric Conductivity
The ability to allow electricity to flow. Electric wires are made of a conductor (copper) coated in an insulator (plastic).
G. Thermal Conductivity
The ability to allow heat to flow. Cooking pans are made of a good conductor (aluminum) while their handles are made of a bad conductor (wood or plastic).
Chemical Properties
These properties describe how a substance reacts with other substances to form new materials.
1. Reactivity
The ability to undergo a chemical reaction. Antacids are used to neutralize stomach acid because their basic properties react with the acid.
2. Flammability
The ability to burn or ignite when exposed to heat. Gasoline's high flammability is used to power car engines.
3. Acidity and Basicity (pH)
Describes whether a substance is an acid, a base, or neutral. Acidic cleaners are used to dissolve mineral buildup (which is basic).
4. Corrosivity
The ability to damage or destroy another material through a chemical reaction. Bridges and cars are painted to prevent corrosion (rusting).
5. Toxicity
The degree to which a substance can damage a living organism. Carbon monoxide detectors are installed in homes to protect against poisoning from this toxic gas.
6. Oxidation
The tendency of a substance to lose electrons, often when combining with oxygen. Antioxidants are added to food to slow down the oxidation that causes spoilage.
7. Radioactivity
The property of an unstable atomic nucleus to spontaneously decay, releasing energy as radiation. In medicine, radiation is used in cancer therapy and medical imaging.
Atoms and Molecules
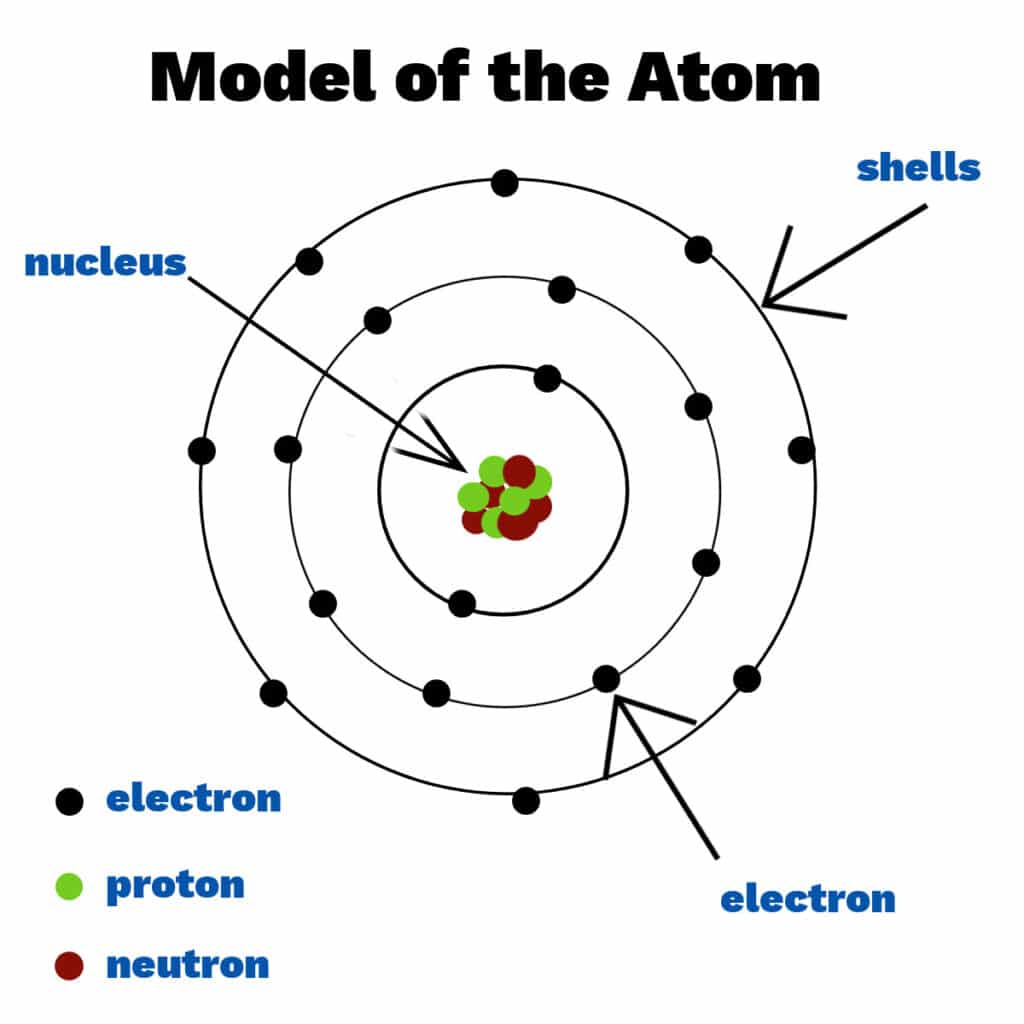
The Atom: The Smallest Chemical Unit
Imagine you have a piece of gold. If you keep cutting it into smaller and smaller pieces, eventually you'd reach the smallest possible piece that still retains the unique characteristics of gold. That irreducible particle is an atom.
An atom is the smallest unit of matter that retains an element's chemical identity.
While it is the smallest chemical unit, it is composed of even smaller, subatomic particles: the electron, proton, and neutron. The central, dense region of an atom is called the nucleus, which holds virtually the entire mass of the atom.
The Structure of an Atom: Subatomic Particles
An atom's properties are dictated by the arrangement and characteristics of its subatomic components:
A. Protons (p+)
Location: Reside in the atom's central core, the nucleus.
Charge: Possess a positive (+) electrical charge.
Significance: The number of protons (the atomic number) is the defining characteristic of an element. Every carbon atom has 6 protons; changing this number changes the element.
B. Neutrons (n0)
Location: Also found within the nucleus.
Charge: Carry no electrical charge (they are neutral).
Significance: Neutrons stabilize the nucleus. The number of neutrons can vary, creating different isotopes of an element (e.g., Carbon-12 vs. the radioactive Carbon-14).
C. Electrons (e−)
Location: Orbit the nucleus in specific energy levels or "shells."
Charge: Possess a negative (-) electrical charge.
Significance: Electrons are the primary mediators of chemical bonding between atoms. Their arrangement in the outermost shell dictates an atom's reactivity.
Analogical Representation: The Atomic Solar System
A helpful, though simplified, analogy for atomic structure is a miniature solar system:
- The nucleus (containing protons and neutrons) is like the sun – a dense, central body with most of the system's mass.
- The electrons are like the planets – smaller entities moving in defined paths around the central mass.
From Atoms to Molecules
While atoms are the fundamental units, matter rarely exists as individual atoms, especially in biological systems.
- Molecules: Atoms combine with other atoms through chemical bonds to form molecules. A molecule is two or more atoms held together by chemical bonds.
- Compounds: When molecules are formed from two or more different types of atoms, they are called compounds.
Examples: An oxygen molecule (O2), a water molecule (H2O), and a glucose molecule (C6H12O6).
Biological Relevance in Nursing
In nursing, understanding how atoms form molecules is critical:
- Medication Action: How drugs bind to receptors involves interactions at the molecular level.
- Physiology: The structure of proteins, carbohydrates, lipids, and nucleic acids – the building blocks of life – are all complex molecules.
- Fluid Balance: Water molecules are paramount in maintaining cellular function and overall homeostasis.
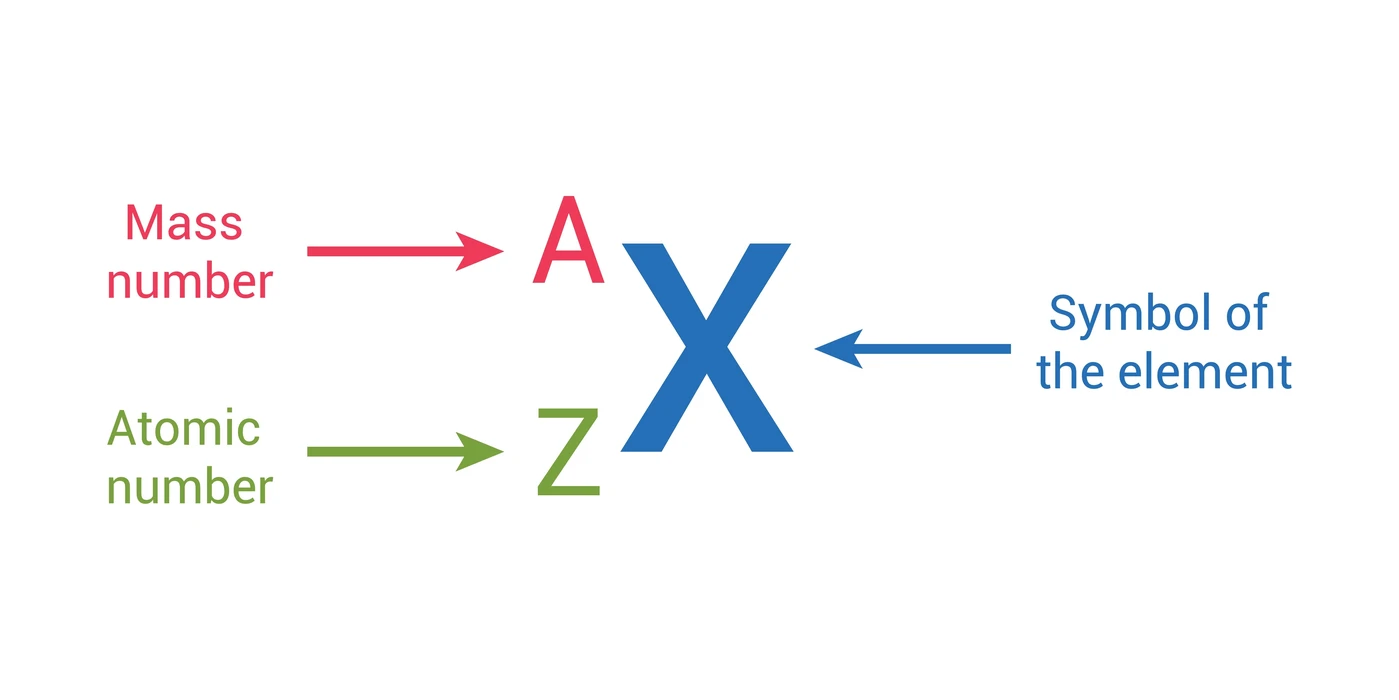
Atomic Number and Mass Number
To precisely characterize any atom and understand its behavior, two fundamental numbers are used: the atomic number and the mass number. These concepts are crucial for interpreting chemical formulas, understanding isotopes, and comprehending atomic stability.
Atomic Number (Z)
Definition: The atomic number (Z) is defined as the exact count of protons residing within an atom's nucleus.
Unique Identifier: This number is the absolute determinant of an element's identity. Each element has a unique atomic number. For example:
- An atom with 1 proton is always Hydrogen (H).
- An atom with 6 protons is always Carbon (C).
- An atom with 8 protons is always Oxygen (O).
Electron Count in Neutral Atoms: For any neutral atom (an atom without an overall electrical charge), the atomic number (number of protons) is precisely equal to the number of electrons.
Mass Number (A)
Definition: The mass number (A) represents the total count of protons and neutrons combined within an atom's nucleus. It essentially provides a measure of the atom's nuclear mass.
Calculation:
Mass Number (A) = Number of Protons + Number of Neutrons
Why Electrons Are Excluded: Electrons are not included because their mass is exceptionally tiny (about 1/1836th of a proton or neutron), making their contribution negligible.
Determining Neutron Count:
Number of Neutrons = Mass Number (A) − Atomic Number (Z)
Illustrative Example: Carbon (C)
Consider a common atom of Carbon (C):
- Its Atomic Number (Z) is 6. This immediately tells us it has 6 protons.
- Its most common Mass Number (A) is 12.
- Using the formula: Number of Neutrons = 12 (Mass Number) − 6 (Atomic Number) = 6 neutrons.
- Therefore, this specific carbon atom has 6 protons, 6 neutrons, and (because it's neutral) 6 electrons.
Isotopes
While all atoms of a specific element share the same number of protons, they can sometimes differ in their neutron count. This variation gives rise to isotopes.
Definition: Isotopes are atoms of the same element (same number of protons) but with different mass numbers (due to a differing number of neutrons).
Analogy: Think of isotopes as siblings within the same family (the element). They share the same parent (the defining number of protons), but they might have different "weights" due to varying numbers of neutrons.
- Chemical Properties: Because they have the same number of protons and electrons, isotopes of an element have nearly identical chemical properties.
- Physical Properties: Due to their different mass numbers, isotopes will have slightly different physical properties, such as density.
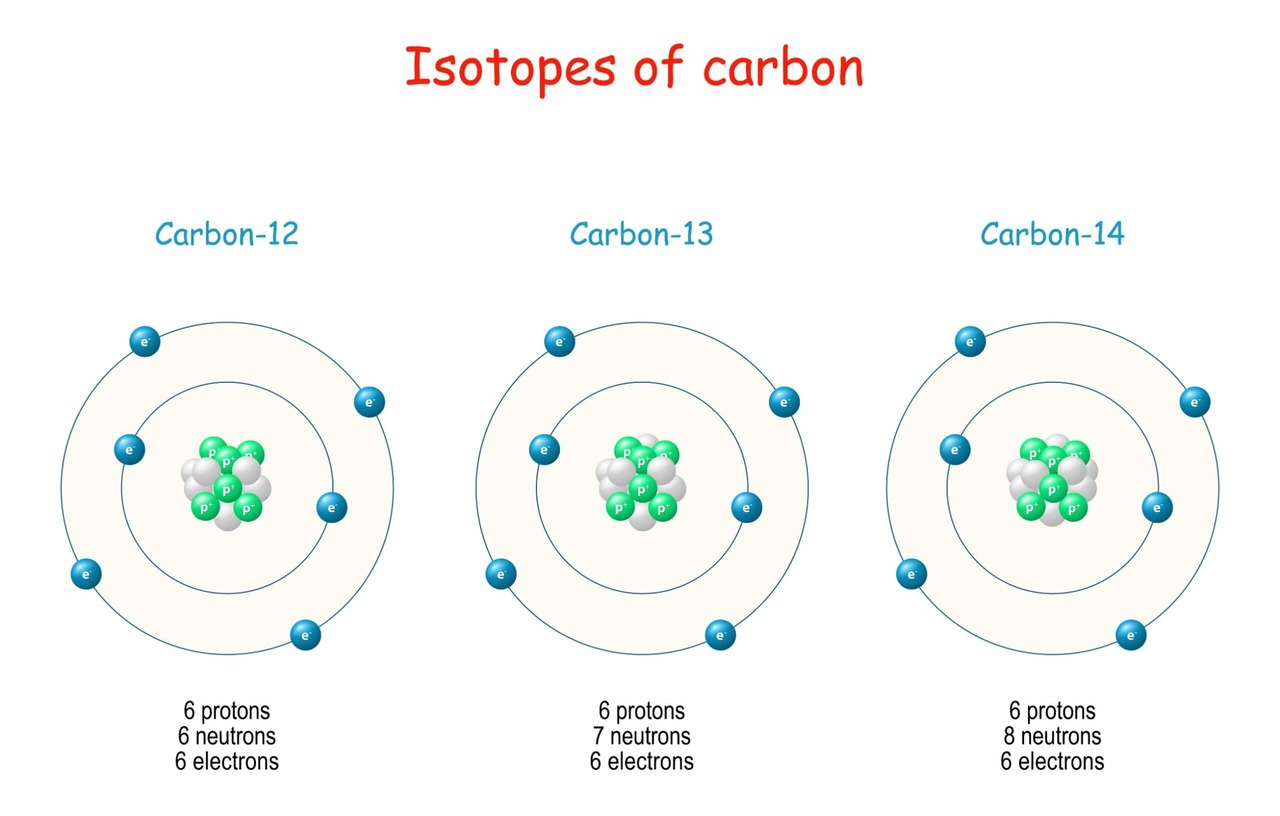
Nomenclature and Examples:
Isotopes are commonly identified by appending their mass number to the element's name.
Carbon Isotopes
All carbon atoms have 6 protons.
- Carbon-12 (12C): 6 protons + 6 neutrons. Most abundant and stable.
- Carbon-13 (13C): 6 protons + 7 neutrons. Stable.
- Carbon-14 (14C): 6 protons + 8 neutrons. Radioactive (used in carbon dating).
Oxygen Isotopes
All oxygen atoms have 8 protons.
- Oxygen-16 (16O): 8 protons + 8 neutrons.
- Oxygen-17 (17O): 8 protons + 9 neutrons.
- Oxygen-18 (18O): 8 protons + 10 neutrons.
Biological and Medical Relevance of Isotopes
Radioactive isotopes (radioisotopes) are invaluable in medicine:
- Medical Imaging and Diagnostics: Technetium-99m is used in bone scans and cardiac stress tests, while Iodine-131 is used to treat or image the thyroid gland.
- Research: Stable isotopes are used as tracers in metabolic studies to track the pathways of molecules within the body.
- Radiation Therapy: Certain radioisotopes are used in controlled doses to target and destroy cancer cells.
Elements and Molecules: Building Complexity
To place isotopes in a broader context, let's briefly revisit the definitions of "element" and "molecule."
What is an Element?
An element is a pure substance composed exclusively of atoms that all share the same number of protons (i.e., the same atomic number). Gold, oxygen, hydrogen, and carbon are prime examples.
What is a Molecule?
A molecule is formed when two or more atoms are held together by specific chemical bonds. If a molecule contains atoms from two or more different elements (like H2O), it is also classified as a compound.
Neutral Atoms vs. Ions
When discussing atoms and molecules, their electrical charge is a critical aspect that influences chemical reactivity and biological function. This discussion will temporarily set aside neutrons, as they do not carry an electrical charge.
Neutral Atoms
Definition: An atom is considered neutral when it possesses no net electrical charge. This is achieved because it contains an equal number of protons (positive charge) and electrons (negative charge).
Number of Protons = Number of Electrons
Example (Neutral Carbon): A carbon atom (Atomic Number 6) is neutral when it has 6 protons (+6 charge) and 6 electrons (−6 charge), resulting in a net charge of 0.
Ions
When an atom is not neutral, it carries a net electrical charge and is termed an ion. Ions are formed when an atom gains or loses electrons during chemical reactions. The number of protons never changes.
1. Cations (Positive Ions)
Formation: A cation forms when an atom loses one or more electrons.
Resulting Charge: By losing negative electrons, the atom is left with more protons than electrons, resulting in an overall positive charge.
Example (Sodium Ion, Na+): Neutral Sodium (Na) has 11 protons and 11 electrons. If it loses 1 electron, it has 11 protons (+11) and 10 electrons (−10), for a net charge of +1.
2. Anions (Negative Ions)
Formation: An anion forms when an atom gains one or more electrons.
Resulting Charge: By gaining negative electrons, the atom has more electrons than protons, resulting in an overall negative charge.
Example (Chloride Ion, Cl−): Neutral Chlorine (Cl) has 17 protons and 17 electrons. If it gains 1 electron, it has 17 protons (+17) and 18 electrons (−18), for a net charge of −1.
The Profound Importance of Ions in Biochemistry and Nursing
The concept of ions is foundational to nearly all biological processes and is critically relevant to nursing practice:
- Nerve Impulse Transmission: Depends on the rapid movement of ions like Na+, K+, and Ca2+.
- Muscle Contraction: Critically dependent on the controlled release of calcium ions (Ca2+).
- pH Regulation: The body's acid-base balance is controlled by hydrogen ions (H+) and bicarbonate ions (HCO3−).
- Fluid and Electrolyte Balance: Electrolytes (ions in solution) are vital for maintaining fluid distribution and cellular function, a common concern in nursing care.
- Enzyme Function: Many enzymes require specific ions as cofactors to function correctly.
In Summary
- Neutral: An atom with an equal number of protons and electrons (no net charge).
- Ion: An atom with an unequal number of protons and electrons (has a net charge).
- Cation: A positively charged ion, formed by losing electrons.
- Anion: A negatively charged ion, formed by gaining electrons.
The Periodic Table: An Organized Map of Elements
The Periodic Table of Elements is an indispensable tool in chemistry and biology, acting as an organized map that classifies all known chemical elements. It reveals patterns and relationships among elements, helping to predict how they might interact in biological systems.
Key Organizational Features for Nurses:
- Groups (Vertical Columns): Elements in the same group often have similar chemical behaviors (e.g., Group 1 elements are all highly reactive).
- Periods (Horizontal Rows): Represent increasing energy levels of atoms.
- Metals vs. Nonmetals: Generally, life-sustaining elements (C, H, N, O, P, S) are found on the nonmetal (right) side, while many vital electrolytes (Na, K, Ca, Mg) are metals found on the left side.
Test Your Knowledge
Biochemistry Lesson One: The Atom.
1. What defines an element's identity?
- Number of neutrons
- Number of electrons
- Number of protons
- Total mass number
Correct (c): The number of protons (atomic number) is the unique "ID card" for each element. If you change the number of protons, you change the element itself.
Incorrect: Neutrons can vary in isotopes, and electrons can be gained or lost to form ions, but the element's identity remains the same.
Analogy: Think of the number of protons as your unique fingerprint. No matter how your weight (neutrons) or clothing (electrons) changes, your fingerprint always identifies you as you.
2. Which subatomic particle has a positive charge?
- Electron
- Neutron
- Proton
- Nucleon
Correct (c): Protons are the subatomic particles that carry a positive (+1) elementary charge.
Incorrect: Electrons are negative, and neutrons are neutral. A nucleon is a general term for particles in the nucleus (protons and neutrons).
Analogy: Remember "Proton" starts with "P," just like "Positive."
3. Where are protons and neutrons located within an atom?
- Electron shells
- Orbitals
- Nucleus
- Outside the atom
Correct (c): The nucleus is the dense, central core of the atom where the protons and neutrons are tightly packed together.
Incorrect: Electron shells and orbitals are the regions where electrons are found, orbiting the nucleus.
Analogy: The nucleus is like the sun in a solar system, with the electrons being the planets orbiting around it.
4. What is the charge of an electron?
- Positive
- Negative
- Neutral
- Varies depending on the atom
Correct (b): Electrons universally carry a negative (-1) elementary charge. This charge is constant and fundamental to the electron.
Incorrect: Protons are positive, and neutrons are neutral. The charge of an electron does not vary.
Analogy: Think of an electron as a tiny magnet that always has its "negative" pole facing out. It can't be changed.
5. An atom has 17 protons, 18 neutrons, and 17 electrons. What is its atomic number?
- 17
- 18
- 35
- 34
Correct (a): The atomic number is defined solely by the number of protons. Since there are 17 protons, the atomic number is 17.
Incorrect: 18 is the neutron count, and 35 (17+18) would be the mass number.
Analogy: The question asks for the atom's "ID number." The number of protons is the only thing that matters for the ID, not the number of neutrons or electrons.
6. For a neutral atom, which of the following is always true?
- Number of protons = Number of neutrons
- Number of electrons = Number of neutrons
- Number of protons = Number of electrons
- Mass number = Atomic number
Correct (c): For an atom to be electrically neutral, the total positive charge from protons must be perfectly balanced by the total negative charge from electrons.
Incorrect: The number of neutrons can vary (isotopes), so it doesn't always equal the number of protons or electrons.
Analogy: A neutral atom is like a balanced seesaw. You have a certain number of positive "weights" (protons) on one side, and you need the exact same number of negative "weights" (electrons) on the other to keep it level.
7. Which subatomic particle is most directly involved in forming chemical bonds?
- Proton
- Neutron
- Electron
- Nucleus
Correct (c): Chemical bonds are formed by the interactions (sharing or transferring) of the outermost electrons between atoms. These are called valence electrons.
Incorrect: Protons and neutrons are locked in the nucleus and do not participate in chemical bonding.
Analogy: Electrons are like the "hands" of an atom. Atoms "hold hands" (form bonds) with each other to form molecules.
8. An atom of Nitrogen (N) has an atomic number of 7 and a mass number of 14. How many neutrons does it have?
- 7
- 14
- 21
- 0
Correct (a): The formula is: Number of neutrons = Mass Number - Atomic Number. So, 14 - 7 = 7 neutrons.
Incorrect: 14 is the mass number (total protons and neutrons).
Analogy: If the total weight of apples and oranges in a bag is 14 (mass number), and you know there are 7 apples (protons), then there must be 7 oranges (neutrons).
9. What is a molecule?
- The smallest unit of an element.
- A substance made of only one type of atom.
- Two or more atoms held together by chemical bonds.
- A subatomic particle with no charge.
Correct (c): This is the definition of a molecule. It can be formed from the same element (like O₂) or different elements (like H₂O).
Incorrect: The smallest unit of an element is an atom. A substance of only one type of atom is an element. A neutral subatomic particle is a neutron.
Analogy: If atoms are individual Lego bricks, a molecule is what you build when you snap two or more bricks together.
10. Atoms of the same element that have different numbers of neutrons are called:
- Ions
- Molecules
- Isotopes
- Allotropes
Correct (c): This is the precise definition of an isotope. They share the same number of protons (same element) but have different masses due to varying neutron counts.
Incorrect: Ions are charged atoms. Molecules are bonded atoms. Allotropes are different structural forms of an element.
Analogy: Isotopes are like different models of the same car. They are all a "Honda Civic" (same protons), but one might be the lightweight model and another the heavier sport model (different neutrons).
11. Which of the following is true about isotopes of an element?
- They have different atomic numbers.
- They have the same number of neutrons.
- They have very similar chemical properties.
- They always have the same mass number.
Correct (c): Chemical properties are determined by electrons, and isotopes have the same number of protons and thus the same number of electrons in their neutral state. This makes their chemical behavior nearly identical.
Incorrect: By definition, isotopes have the same atomic number but different numbers of neutrons and therefore different mass numbers.
Analogy: Different models of the same car (isotopes) will all drive on the same roads and use the same type of fuel (similar chemical properties) even though they have different weights.
12. Carbon-14 is a radioactive isotope of Carbon. Compared to Carbon-12, Carbon-14 has:
- More protons
- Fewer protons
- More neutrons
- Fewer electrons
Correct (c): Both are Carbon, so they must have 6 protons. Carbon-14 has a mass of 14 (6 protons + 8 neutrons). Carbon-12 has a mass of 12 (6 protons + 6 neutrons). Therefore, Carbon-14 has two more neutrons.
Incorrect: Changing the number of protons would change the element from Carbon to something else.
Analogy: Both are "Carbon" cars, but Carbon-14 has extra luggage in the trunk (more neutrons) compared to Carbon-12.
13. What is the approximate mass of an electron compared to a proton or neutron?
- About the same
- Much larger
- Much smaller
- Exactly double
Correct (c): The mass of an electron is about 1/1836th the mass of a proton, making its contribution to an atom's total mass negligible.
Incorrect: Protons and neutrons have similar masses, but electrons are vastly lighter.
Analogy: If a proton is a bowling ball, an electron is a grain of sand. The sand adds almost no noticeable weight to the overall mass.
14. If an atom has an atomic number of 11 and a mass number of 23, how many electrons does it have in its neutral state?
- 11
- 12
- 23
- 34
Correct (a): In a neutral atom, the number of electrons equals the number of protons. The atomic number tells us there are 11 protons, so there must be 11 electrons.
Incorrect: 12 is the number of neutrons (23 - 11), and 23 is the mass number.
Analogy: For the seesaw to be balanced (neutral), you need 11 positive "weights" (protons) and 11 negative "weights" (electrons).
15. Which statement best describes the role of the number of protons in an atom?
- It determines the atom's stability.
- It determines the atom's reactivity.
- It determines the element's identity.
- It determines the atom's overall size.
Correct (c): The atomic number (number of protons) is the unique identifier for each element. It's the most fundamental property that defines what an element is.
Incorrect: Stability also depends on neutrons, reactivity on electrons, and size on electron shells.
Analogy: The number of protons is the atom's non-negotiable "last name." Changing it means you're talking about a completely different family (element).
16. The dense, central part of an atom containing protons and neutrons is called the __________.
17. Atoms of the same element must always have the same number of __________.
18. The sum of protons and neutrons in an atom's nucleus is known as the __________.
19. Negatively charged subatomic particles that orbit the nucleus are called __________.
20. Isotopes differ in their number of __________.
Quiz Complete!
Your Score:
0%
0 / 0 correct