Nerve and Muscle Physiology:Basis and Application
Nerve and Muscle Physiology
Nerve and muscle physiology is a branch of physiology that specifically studies the function and mechanisms of nervous tissue (nerves) and muscle tissue (muscles).
It explores how these "excitable tissues" generate and transmit electrical signals (like action potentials) and how these electrical signals are converted into specific cellular functions.
For Nerves:
It covers how neurons (nerve cells) generate electrical impulses, communicate with each other (synaptic transmission), process information, and transmit signals throughout the body to control various functions, from thought and sensation to movement and organ regulation.
For Muscles:
It focuses on how muscle cells (fibers) respond to electrical signals from nerves, leading to contraction (shortening) and the generation of force. This includes the molecular mechanisms of contraction, the regulation of muscle force, and the different types of muscle tissue and their distinct functional characteristics.
Nervous System Excitability
Nervous system excitability is the ability of nerve cells (neurons) to respond to a stimulus by generating and propagating an action potential, a self-propagating electrical impulse.
This property is fundamental to the nervous system's function and depends on the neuron's membrane's selective permeability, ion channels, and pumps. A change in membrane potential can lead to this event, which is essential for transmitting information throughout the body. The physiology of the nervous system involves its main divisions (the Central Nervous System (CNS) and Peripheral Nervous System (PNS)), which use neurons and electrochemical signals to sense stimuli, integrate information, and produce coordinated responses.
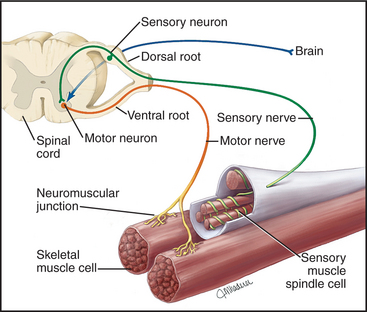
Overall Structure & Function of a Motor Neuron (The Command Pathway)
A motor neuron is a specialized nerve cell that transmits electrical signals from the central nervous system (brain and spinal cord) to muscles or glands, thereby initiating movement or secretion. It acts as the "final common pathway" by which the nervous system controls effector organs.
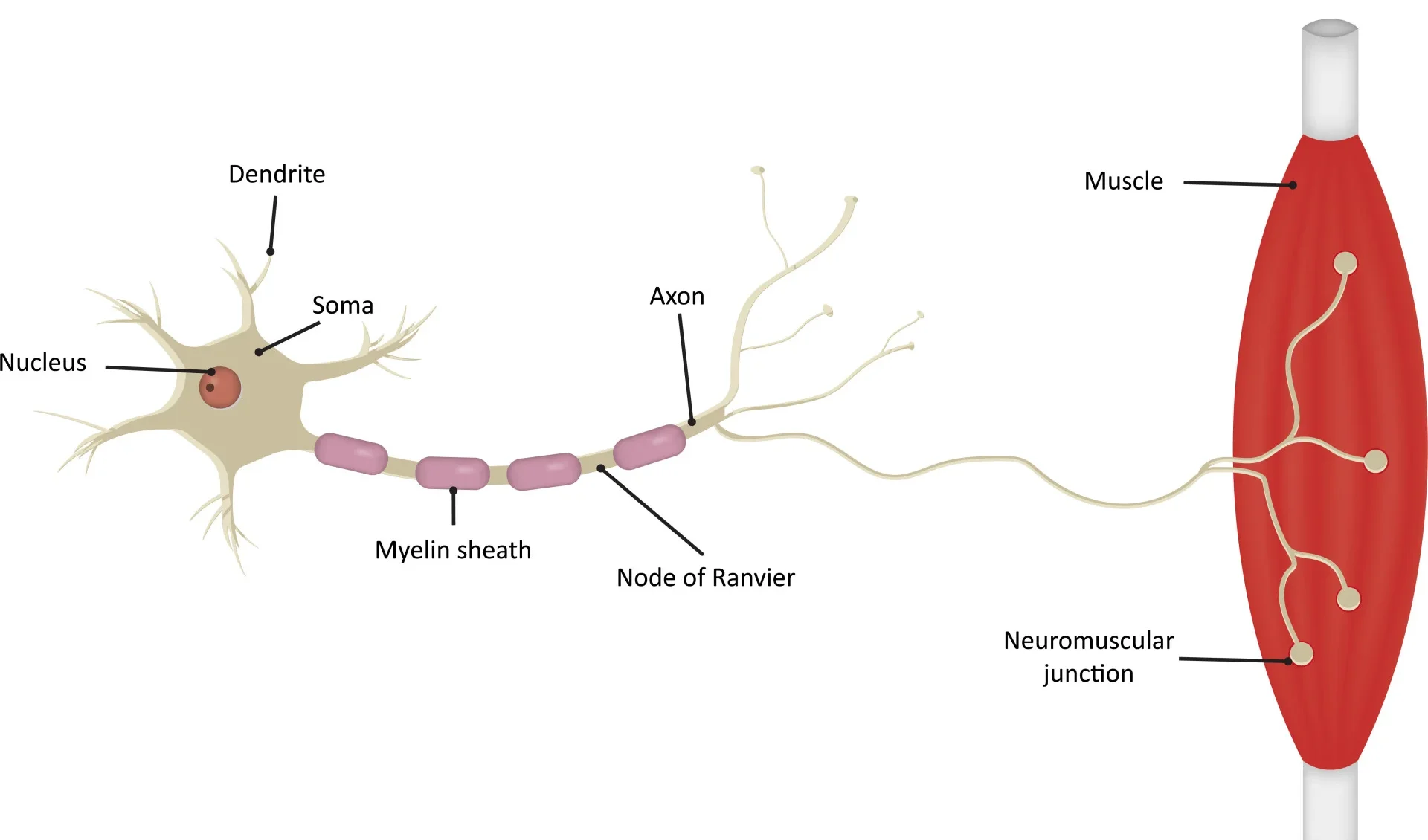
1. Motor Neuron Anatomy: Key Structural Components
Cell Body (Soma/Perikaryon)
The metabolic center of the neuron, containing the nucleus and other organelles. It synthesizes neurotransmitters and proteins and receives synaptic inputs from other neurons.
Dendrites
Branching, tree-like extensions that are the primary receptive (input) regions. They contain ligand-gated ion channels that receive chemical signals and generate graded potentials (EPSPs and IPSPs).
Axon Hillock
A cone-shaped region where the axon originates. This is the critical "trigger zone" with the highest density of voltage-gated Na⁺ channels. It integrates all incoming potentials, and if the sum reaches threshold, an action potential is generated.
Axon
A single, long projection that transmits the action potential (the output signal) away from the cell body. Its length can exceed a meter.
Myelin Sheath
A fatty, insulating layer that surrounds many axons, formed by Schwann cells in the PNS and oligodendrocytes in the CNS. It is crucial for increasing the speed of action potential conduction.
Nodes of Ranvier
Gaps in the myelin sheath that contain a high concentration of voltage-gated Na⁺ and K⁺ channels. The action potential is regenerated at these nodes, "jumping" from one to the next in a process called saltatory conduction.
Axon Terminals (Synaptic Terminals)
The branched ends of the axon that form synapses with other cells. They contain synaptic vesicles filled with neurotransmitters and are specialized for converting the electrical signal (action potential) into a chemical signal (neurotransmitter release).
2. Functional Zones: Relating Structure to Role
We can map these anatomical components to four distinct functional zones, illustrating the flow of information:
3. Role in Motor Control: The Final Common Pathway
Motor neurons are often referred to as the "final common pathway" in motor control. This term emphasizes a fundamental principle: all the complex neural computations happening in higher brain centers (e.g., planning and coordination in the cerebral cortex, basal ganglia, and cerebellum) ultimately converge onto these lower motor neurons.
It is only through the firing of a lower motor neuron that a skeletal muscle can be activated and a movement can occur. Regardless of whether a movement is voluntary or reflexive, the command signal ultimately travels down a lower motor neuron to its target muscle fibers. This makes the motor neuron a critical bottleneck and the ultimate determinant of muscle activity and all bodily movements.
Synaptic Transmission (The Communication Bridge Between Neurons)
Synaptic transmission is the fundamental process by which one neuron (the presynaptic neuron) communicates with another neuron (the postsynaptic neuron) or an effector cell. Most synapses in the nervous system are chemical synapses, meaning they utilize chemical messengers called neurotransmitters to bridge the microscopic gap between cells.
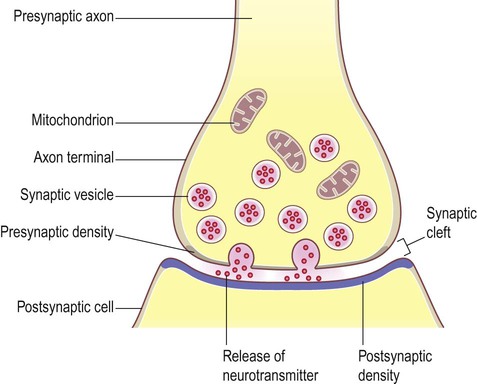
Anatomy of a Chemical Synapse
A chemical synapse consists of three main components:
- Presynaptic Terminal (Axon Terminal): The specialized end of the presynaptic axon. It contains synaptic vesicles filled with neurotransmitters, abundant mitochondria for energy, and crucial voltage-gated Ca²⁺ channels.
- Synaptic Cleft: The microscopic, fluid-filled space (typically 20-50 nm wide) that separates the presynaptic and postsynaptic membranes.
- Postsynaptic Membrane: The specialized region of the receiving cell's membrane, containing a high density of specific neurotransmitter receptors.
Neurotransmitter Synthesis & Storage
Neurotransmitters are synthesized via distinct pathways and then packaged into synaptic vesicles. This packaging protects them from degradation, concentrates them for efficient release, and ensures their availability.
Presynaptic Events: Neurotransmitter Release
This phase converts the electrical signal into a chemical signal:
- Action Potential Arrives: An action potential propagates down the axon and depolarizes the presynaptic terminal.
- Depolarization Opens Voltage-Gated Ca²⁺ Channels: The change in membrane potential activates and opens these channels.
- Ca²⁺ Influx: Due to a steep electrochemical gradient, Ca²⁺ ions rapidly rush into the presynaptic terminal. This influx is the essential trigger for neurotransmitter release.
- Ca²⁺ Triggers Vesicle Fusion: The increase in intracellular Ca²⁺ causes synaptic vesicles to fuse with the presynaptic membrane, mediated by SNARE proteins.
- Neurotransmitter Release (Exocytosis): As vesicles fuse, neurotransmitters are rapidly expelled into the synaptic cleft.
Postsynaptic Events: Receptor Binding & Ion Channel Opening
Once in the cleft, neurotransmitters diffuse across and bind reversibly to their specific receptors on the postsynaptic membrane, causing a response.
Ligand-Gated Ion Channels (Ionotropic)
The receptor itself is an ion channel. Binding of the neurotransmitter causes an immediate opening, allowing ion flow and a rapid change in the postsynaptic membrane potential. This can generate:
- Excitatory Postsynaptic Potential (EPSP): Depolarization (e.g., via Na⁺ influx), making the neuron more likely to fire.
- Inhibitory Postsynaptic Potential (IPSP): Hyperpolarization (e.g., via Cl⁻ influx or K⁺ efflux), making the neuron less likely to fire.
G-Protein Coupled Receptors (Metabotropic)
The receptor activates an intracellular G-protein, which then initiates a slower but more widespread and long-lasting signaling cascade. This can lead to:
- Direct modulation of nearby ion channels.
- Production of "second messengers" (e.g., cAMP) that can alter protein synthesis or gene expression.
These events generate graded potentials (EPSPs or IPSPs). If the combined effect of these graded potentials at the axon hillock reaches threshold, a new action potential is triggered in the postsynaptic neuron.
Neurotransmitter Inactivation/Removal: Terminating the Signal
To ensure precise and discrete signaling, the action of neurotransmitters must be swiftly terminated. This happens through several mechanisms:
- Enzymatic Degradation: Specific enzymes in the synaptic cleft break down the neurotransmitter. The classic example is acetylcholinesterase (AChE) breaking down acetylcholine.
- Reuptake: Specialized transporter proteins on the presynaptic terminal (or nearby glial cells) actively pump the neurotransmitter back into the cell for recycling. This is the primary mechanism for monoamines like serotonin, dopamine, and norepinephrine.
- Diffusion: Some neurotransmitters simply diffuse away from the synaptic cleft, where their concentration drops and their effect is diminished.
Generation of a Motor Neuron Action Potential
The motor neuron is constantly bombarded with chemical signals from thousands of other neurons. These signals cause small, localized changes in the membrane potential, which the neuron must integrate to decide whether to fire an "all-or-nothing" action potential.
Synaptic Input: EPSPs and IPSPs
When a presynaptic neuron releases neurotransmitters, they bind to ligand-gated ion channels on the motor neuron, leading to a change in its membrane potential.
Excitatory Postsynaptic Potential (EPSP)
A depolarization of the postsynaptic membrane, making it less negative and more likely to fire. Typically caused by the influx of positive ions, most commonly Na⁺, when an excitatory neurotransmitter (e.g., glutamate) binds.
Inhibitory Postsynaptic Potential (IPSP)
A hyperpolarization or stabilization of the membrane potential, making it more negative and less likely to fire. Typically caused by the influx of negative ions (Cl⁻) or the efflux of positive ions (K⁺) when an inhibitory neurotransmitter (e.g., GABA, glycine) binds.
Spatial and Temporal Summation
A single EPSP is usually too weak to trigger an action potential. Motor neurons integrate thousands of inputs:
- Spatial Summation: Multiple EPSPs or IPSPs arriving at different locations simultaneously can add together.
- Temporal Summation: Rapid, successive EPSPs or IPSPs from a single presynaptic neuron can add up over time.
The axon hillock acts as the integrator. If the algebraic sum of all incoming EPSPs and IPSPs reaches the threshold potential (typically around -55 mV), an action potential is generated.
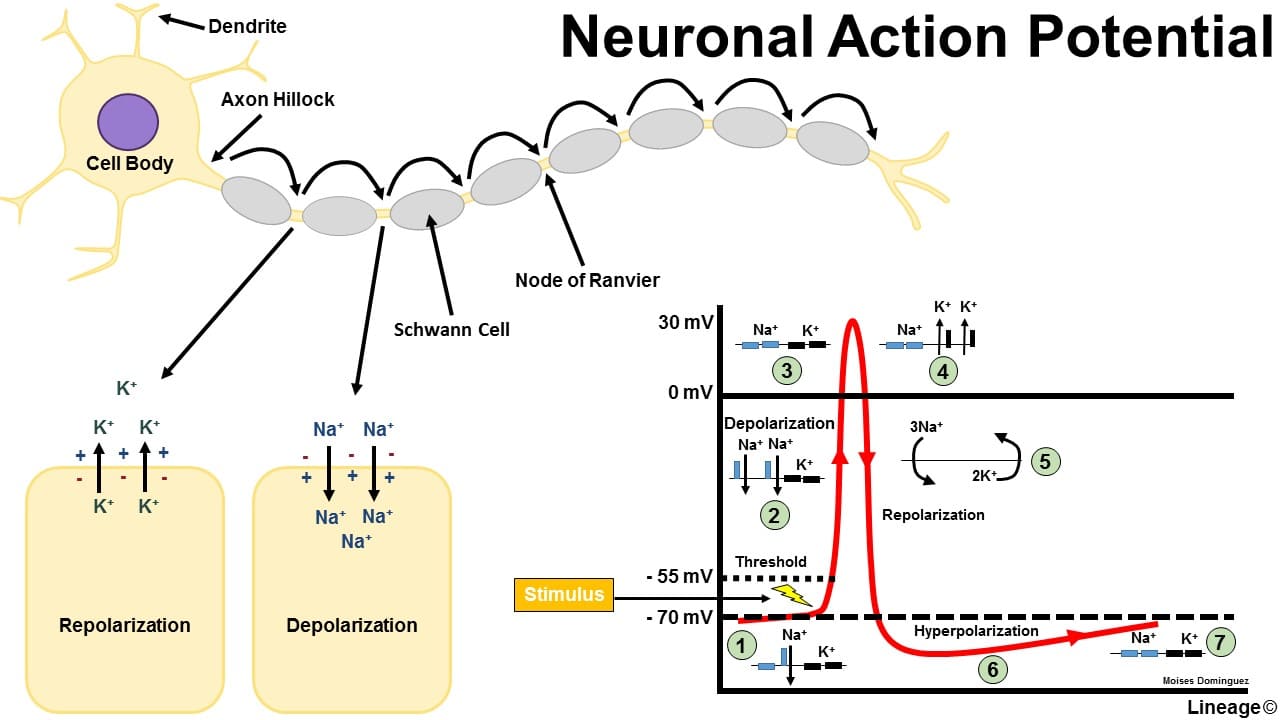
Conduction of the Motor Neuron Action Potential
Once generated at the axon hillock, the action potential propagates along the axon without losing strength.
Action Potential Phases (in a Motor Neuron):
- Resting State (-70 mV): The membrane is polarized. All voltage-gated Na⁺ and K⁺ channels are closed. The RMP is maintained by K⁺ leak channels and the Na⁺/K⁺-ATPase pump.
- Depolarization to Threshold (-55 mV): The summed EPSPs cause a localized depolarization. If it reaches threshold, the positive feedback loop for Na⁺ channel activation begins.
- Rising Phase (Rapid Depolarization to +30mV): At threshold, voltage-gated Na⁺ channels rapidly open. A massive influx of Na⁺ causes a swift and strong depolarization, making the inside of the membrane positive.
- Falling Phase (Repolarization): At the peak, voltage-gated Na⁺ channels inactivate (stopping Na⁺ influx), and the slower voltage-gated K⁺ channels fully open. A large efflux of K⁺ rapidly repolarizes the membrane.
- Undershoot/Hyperpolarization (below -70 mV): The slow-to-close voltage-gated K⁺ channels cause an excessive efflux of K⁺, making the membrane briefly more negative than the RMP. This phase is critical for the refractory periods:
- Absolute Refractory Period: During the rising and initial falling phase, no stimulus can generate another action potential because Na⁺ channels are either open or inactivated. This ensures one-way propagation.
- Relative Refractory Period: During the undershoot phase, a stronger-than-normal stimulus is required to generate another action potential because the membrane is hyperpolarized.
- Restoration of Resting Potential: All voltage-gated channels close. The Na⁺/K⁺-ATPase pump works continuously in the background to restore and maintain the long-term ion concentration gradients.
Muscle Physiology: Contraction and Relaxation
Muscle tissue is specialized for contraction, generating force and movement. Here, we'll focus on skeletal muscle.
A. Skeletal Muscle Structure
- Muscle Fiber (Cell): A single, elongated, multinucleated cell.
- Sarcolemma: The specialized plasma membrane of a muscle fiber, with invaginations called T-tubules.
- Sarcoplasm: The cytoplasm of a muscle fiber, containing mitochondria, glycogen, myoglobin, and myofibrils.
- Myofibrils: Long, rod-like contractile organelles composed of repeating sarcomeres.
- Sarcoplasmic Reticulum (SR): A specialized smooth ER that surrounds each myofibril, storing and releasing Ca²⁺ ions.
- T-Tubules (Transverse Tubules): Deep invaginations of the sarcolemma that conduct action potentials into the cell's interior. A triad consists of a T-tubule flanked by two terminal cisternae of the SR.
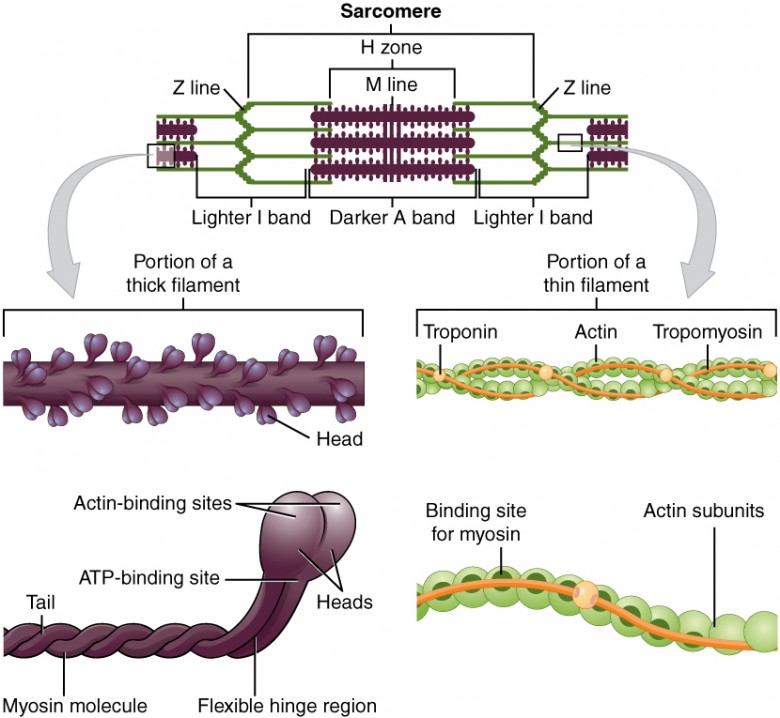
B. The Sarcomere: The Contractile Unit
The sarcomere is the fundamental, repeating contractile unit of a myofibril, extending from one Z-disc to the next.
Filaments:
- Thick Filaments (Myosin): Composed of myosin protein. Each molecule has a tail and two globular heads. The heads contain an actin-binding site and an ATP-binding site (which also functions as an ATPase).
- Thin Filaments (Actin): Composed primarily of actin. Also contain two crucial regulatory proteins:
- Tropomyosin: A rod-shaped protein that covers the myosin-binding sites on actin in a relaxed muscle.
- Troponin: A complex of three proteins. Troponin C (TnC) is the component that binds Ca²⁺ ions, initiating contraction.
Bands and Zones:
- A Band: The entire length of the thick filament (dark). Its length remains constant during contraction.
- I Band: Contains only thin filaments (light). It shortens during contraction.
- H Zone: The central region of the A band with only thick filaments. It shortens during contraction.
- M Line: A dark line in the center of the H zone that anchors thick filaments.
- Z Disc (Z Line): Defines the ends of a sarcomere and anchors the thin filaments.

The Neuromuscular Junction (The Link from Nerve to Muscle)
The neuromuscular junction (NMJ) is the specialized chemical synapse where a motor neuron's axon terminal meets a skeletal muscle fiber.
Anatomy of the NMJ:
- Presynaptic Terminal: The end of the motor neuron's axon, containing synaptic vesicles filled with acetylcholine (ACh).
- Synaptic Cleft: The space between the nerve and muscle, containing the enzyme acetylcholinesterase (AChE).
- Motor End Plate: A specialized region of the sarcolemma with junctional folds packed with acetylcholine receptors (AChRs).
Neurotransmitter & Receptor: Acetylcholine's Role
- Acetylcholine (ACh): The sole neurotransmitter used to excite skeletal muscle.
- ACh Receptors (nAChRs): These are ligand-gated ion channels on the motor end plate. When two ACh molecules bind, the channel opens, allowing both Na⁺ and K⁺ to pass through.
End-Plate Potential (EPP): The Muscle's First Electrical Response
This is the muscle's initial, graded electrical response at the motor end plate:
- ACh Release: An action potential in the motor neuron triggers the release of ACh into the synaptic cleft.
- ACh Binding: ACh diffuses across the cleft and binds to AChRs on the motor end plate.
- Channel Opening: The binding of two ACh molecules opens the ion channel.
- Ion Movement: Na⁺ ions rapidly rush into the muscle fiber, while a smaller amount of K⁺ ions move out. The net effect is a significant influx of positive charge.
- Depolarization (EPP): This net influx of positive ions causes a rapid, large, localized depolarization of the motor end plate, known as the End-Plate Potential (EPP). An EPP is always large enough to trigger an action potential in the adjacent sarcolemma.
- ACh Inactivation: ACh is rapidly degraded by acetylcholinesterase (AChE) in the synaptic cleft, terminating the signal and allowing the muscle fiber to repolarize.
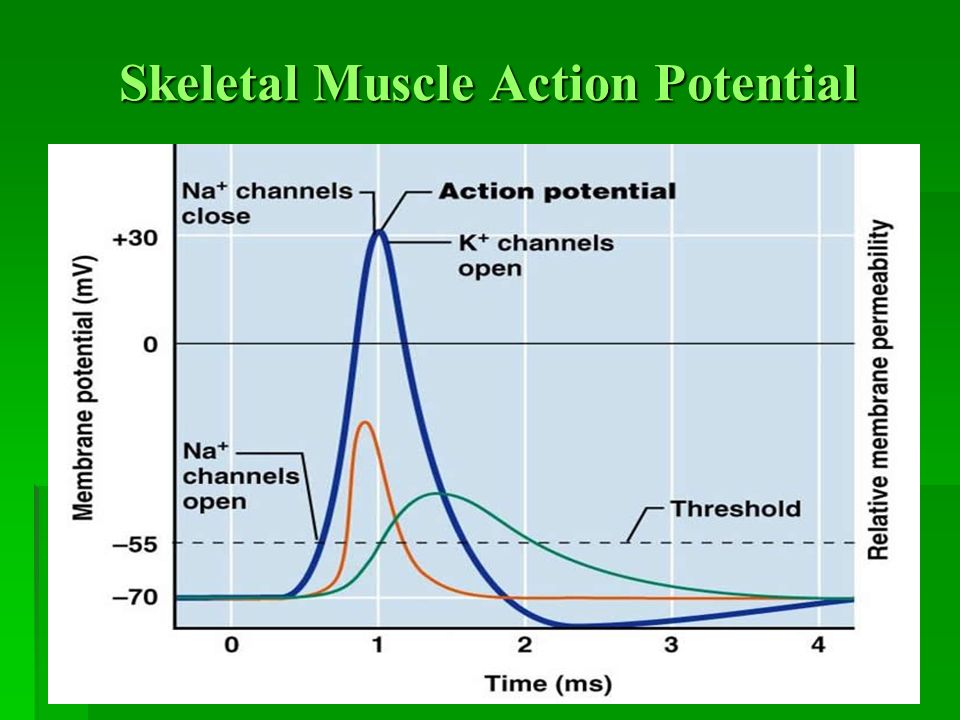
The Muscle Action Potential (Electrical Signal within the Muscle Cell)
The muscle action potential is an "all-or-nothing" electrical signal that rapidly spreads across the entire muscle fiber membrane. Its characteristics are very similar to the neuronal action potential, but its purpose is specifically to initiate muscle contraction.
Propagation: Spreading the Signal Deep Within
The muscle action potential propagates in two critical ways:
- Along the Sarcolemma: Spreading in both directions along the length of the muscle fiber.
- Into the T-tubules: The action potential rapidly dives down into these deep invaginations of the sarcolemma. This is crucial because it brings the electrical signal into very close proximity with the sarcoplasmic reticulum (SR), which stores the Ca²⁺ needed for contraction.
Excitation-Contraction Coupling
This is the physiological process by which an electrical signal (the muscle action potential) is converted into a mechanical event (muscle contraction).
- Muscle AP Propagation: The action potential travels along the sarcolemma and down into the T-tubules.
- DHPR Activation: The action potential causes a conformational change in voltage-sensitive proteins in the T-tubule membrane called Dihydropyridine Receptors (DHPRs).
- Mechanical Linkage to RyRs: The DHPRs are mechanically linked to Ryanodine Receptors (RyRs), which are Ca²⁺ release channels on the sarcoplasmic reticulum (SR).
- RyR Opening and Ca²⁺ Release: The change in the DHPRs mechanically pulls open the RyRs, allowing stored Ca²⁺ ions to flood out of the SR and into the sarcoplasm.
- Increase in Intracellular Ca²⁺: This rapid increase in sarcoplasmic Ca²⁺ concentration is the immediate trigger for muscle contraction.
The Mechanism of Muscle Contraction (The "Sliding Filament Theory")
The Sliding Filament Theory proposes that muscle shortening occurs by the thick and thin filaments sliding past one another, thereby increasing their overlap.
1. Role of Ca²⁺: Unlocking the Binding Sites
- Ca²⁺ Binds to Troponin C: Ca²⁺ ions released from the SR bind to the Troponin C subunit on the thin filaments.
- Tropomyosin Shifts: This binding causes a shape change in troponin, which in turn tugs on the tropomyosin molecule.
- Active Sites Exposed: The movement of tropomyosin physically shifts it away from the myosin-binding sites on the actin molecules, which were previously blocked.
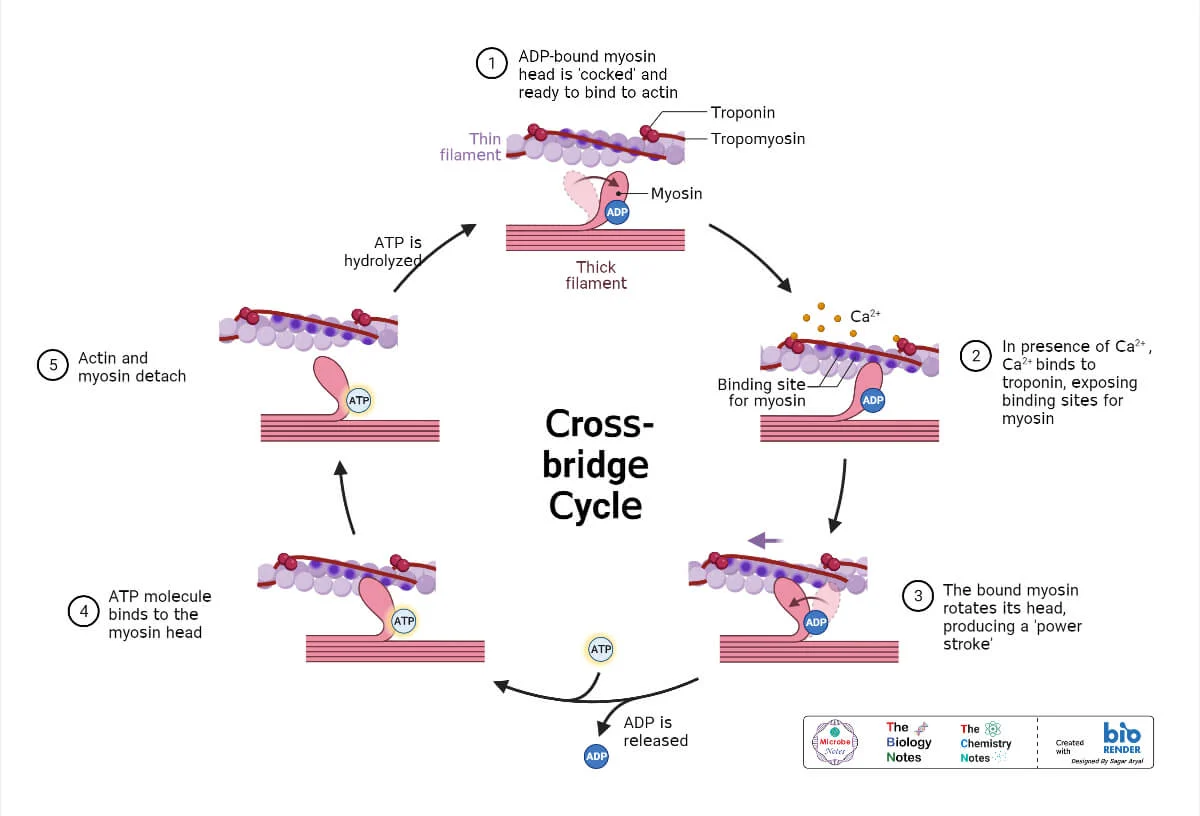
Cross-Bridge Cycle (Molecular Events): The Powerhouse
The cross-bridge cycle is a repetitive series of events that causes the thin filaments to slide over the thick filaments.
- The thin filaments to slide inward, past the stationary thick filaments.
- The Z-discs to be pulled closer together, shortening the entire sarcomere.
- The I bands and H zone to shorten.
- The A band to remain unchanged in length.
- Cessation of Motor Neuron Signal: The motor neuron stops firing, and no more ACh is released.
- AChE Activity: Remaining ACh in the synaptic cleft is rapidly broken down by acetylcholinesterase.
- Repolarization of Sarcolemma: The muscle fiber action potential ceases.
- Ca²⁺ Reuptake into SR: As the T-tubules repolarize, the RyRs on the SR close. Simultaneously, active transport pumps called SERCA pumps use ATP to actively pump Ca²⁺ from the sarcoplasm back into the SR.
- Tropomyosin Blocks Active Sites: As sarcoplasmic Ca²⁺ levels fall, Ca²⁺ detaches from Troponin C. Troponin returns to its original shape, allowing tropomyosin to shift back and cover the myosin-binding sites on actin.
- Muscle Relaxes: With cross-bridge formation prevented, the muscle fiber passively lengthens or remains at its resting length.
Step 1: Cross-Bridge Formation
The energized ("cocked") myosin head, which is already holding onto ADP and inorganic phosphate (Pi) from the previous cycle, has a strong chemical attraction (affinity) for the actin filament. This binding can only occur if the myosin-binding sites on the actin are exposed. Once the sites are uncovered by the movement of tropomyosin (triggered by Ca²⁺ binding to troponin), the myosin head immediately forms a strong physical link with the actin. This connection is the "cross-bridge."
Step 2: The Power Stroke
The formation of the cross-bridge triggers the release of the inorganic phosphate (Pi) from the myosin head. This release unleashes the stored energy, causing the myosin head to pivot forcefully from its high-energy 90° angle to a low-energy 45° angle. This pivotal movement is the power stroke. Because it is firmly attached, the myosin head drags the entire thin filament a short distance (~10 nm) toward the center of the sarcomere. Immediately after the pivot, the ADP molecule is also released, leaving the myosin head in a low-energy state, still tightly bound to actin.
Step 3: Cross-Bridge Detachment
After the power stroke, the myosin head is "stuck" to the actin in a low-energy state (the "rigor" state). The only way for it to let go is for a new molecule of ATP to bind to the ATP-binding site on the myosin head. This binding causes a conformational change that weakens the bond between myosin and actin, reducing their affinity for each other and causing the myosin head to detach. Without a fresh supply of ATP, this detachment cannot occur, which is the molecular basis for the muscle stiffness seen in rigor mortis after death.
Step 4: Re-cocking of the Myosin Head
The myosin head, now with ATP bound, immediately acts as an enzyme (myosin ATPase) and hydrolyzes the ATP back into ADP and inorganic phosphate (Pi). The energy released from breaking this ATP bond is captured by the myosin head and used to change its shape, moving it from its low-energy bent position back to its high-energy, upright, "cocked" position. It is now energized and reset, ready to begin the cycle again by binding to another active site further down the actin filament (if Ca²⁺ is still present).
4. Sarcomere Shortening: The Result of Sliding Filaments
Repeated cycles of the cross-bridge cycle cause:
When thousands of sarcomeres shorten simultaneously, the entire muscle shortens and generates force.
Muscle Relaxation
Muscle relaxation is an active, energy-requiring process.
Test Your Knowledge
A quiz covering Nerve and Muscle Physiology.
1. Which of the following is the primary role of the T-tubules in skeletal muscle contraction?
- Store calcium ions
- Synthesize ATP for muscle contraction
- Conduct action potentials deep into the muscle fiber
- Anchor thin filaments in the sarcomere
Correct (c): T-tubules conduct action potentials from the sarcolemma surface deep into the muscle fiber, ensuring simultaneous activation of all myofibrils.
Incorrect: Ca2+ storage is by the SR, ATP synthesis by mitochondria, and thin filament anchoring by Z-discs.
Analogy: Think of T-tubules as a subway system quickly delivering an important message (action potential) to all neighborhoods (myofibrils) within the muscle city.
2. Which ion's rapid influx into the motor neuron terminal triggers the release of acetylcholine (ACh)?
- Sodium (Na+)
- Potassium (K+)
- Calcium (Ca2+)
- Chloride (Cl-)
Correct (c): Influx of extracellular Ca2+ into the presynaptic terminal acts as the signal that triggers the fusion of ACh-containing vesicles with the presynaptic membrane.
Analogy: Ca2+ is like the "go-ahead" button for vesicles to release their neurotransmitter payload.
Incorrect: Na+ is for AP depolarization, K+ for repolarization, and Cl- for inhibition.
3. What is the primary function of acetylcholinesterase (AChE) at the neuromuscular junction?
- To synthesize new acetylcholine molecules
- To transport acetylcholine back into the presynaptic terminal
- To break down acetylcholine in the synaptic cleft
- To bind to acetylcholine receptors and open ion channels
Correct (c): AChE rapidly degrades ACh in the synaptic cleft, terminating the signal and allowing the muscle to relax and prepare for the next impulse.
Analogy: AChE is like a cleanup crew removing the "message" (ACh) from the bulletin board (receptor) promptly.
Incorrect: ACh synthesis and receptor binding are distinct processes; AChE's role is degradation.
4. The End-Plate Potential (EPP) at the neuromuscular junction is primarily caused by the net movement of which ions?
- Na+ out, K+ in
- K+ out, Na+ in
- Ca2+ out, K+ in
- Na+ in, K+ out
Correct (d): ACh opens non-selective cation channels. More Na+ rushes in than K+ leaves, causing a net influx of positive charge and depolarization (EPP).
Incorrect: The directions of ion movement are wrong or the primary ion is incorrect.
5. What is the direct consequence of Ca2+ binding to Troponin C in skeletal muscle?
- Myosin heads hydrolyze ATP
- Tropomyosin moves, exposing actin-binding sites
- Myosin heads detach from actin
- The sarcoplasmic reticulum reabsorbs Ca2+
Correct (b): Ca2+ binding to Troponin C causes a conformational change that pulls tropomyosin away from the myosin-binding sites on actin, allowing cross-bridge formation.
Analogy: Ca2+ is like a key that unlocks a protective shield (tropomyosin) covering the active sites.
Incorrect: ATP binding causes detachment; ATP hydrolysis cocks the myosin; Ca2+ reuptake occurs during relaxation.
6. During the power stroke, which event immediately follows the binding of the myosin head to actin?
- ATP binds to the myosin head
- Pi (inorganic phosphate) is released from the myosin head
- The myosin head re-cocks
- Ca2+ is reabsorbed into the SR
Correct (b): The sequence is: energized myosin (ADP+Pi) binds actin -> Pi is released -> Power stroke (ADP released) -> ATP binds causing detachment.
Incorrect: ATP binding causes detachment, Pi release triggers the power stroke, and Ca2+ reuptake is for relaxation.
7. Which component of the sarcomere remains unchanged in length during muscle contraction?
- I band
- H zone
- A band
- Sarcomere length
Correct (c): The A band corresponds to the length of the thick filament, which does not shorten; thin filaments slide over it.
Incorrect: I band, H zone, and sarcomere length all shorten during contraction.
8. Which statement about the role of ATP in muscle contraction is TRUE?
- ATP is directly used to move tropomyosin off actin.
- ATP binding to myosin causes its detachment from actin.
- ATP hydrolysis directly powers the power stroke.
- ATP is only required for relaxation.
Correct (b): A new ATP molecule must bind to the myosin head to reduce its affinity for actin, allowing detachment.
Incorrect: Ca2+ moves tropomyosin; ATP hydrolysis energizes myosin for the power stroke after binding; ATP is crucial for both contraction and relaxation.
9. What is the primary role of voltage-gated Ca2+ channels in the motor neuron terminal?
- Initiate the action potential in the motor neuron.
- Cause the repolarization of the motor neuron terminal.
- Trigger the release of neurotransmitter into the synaptic cleft.
- Generate the end-plate potential in the muscle fiber.
Correct (c): When the action potential arrives, it opens these channels, allowing Ca2+ influx which signals synaptic vesicles to release ACh.
Incorrect: Action potentials are initiated by Na+ channels; repolarization by K+ channels; EPPs are on the muscle fiber.
10. Blocking Ryanodine Receptors (RyRs) on the SR would directly prevent:
- Acetylcholine release from the motor neuron.
- The generation of a muscle action potential.
- The release of Ca2+ into the sarcoplasm.
- The reuptake of Ca2+ into the SR during relaxation.
Correct (c): RyRs are the Ca2+ release channels on the SR. Blocking them prevents Ca2+ from escaping the SR into the sarcoplasm, thus halting contraction.
Incorrect: ACh release is presynaptic; muscle APs are on the sarcolemma; Ca2+ reuptake is by SERCA pumps.
11. Why is the action potential in a motor neuron considered "all-or-nothing"?
- Because it travels only in one direction.
- Because it either fires at full strength or not at all, once threshold is reached.
- Because it requires all ion channels to open simultaneously.
- Because it only occurs at the Nodes of Ranvier.
Correct (b): If the threshold is reached, a full-sized action potential occurs; if not, none occurs. Its amplitude is constant, independent of stimulus strength beyond threshold.
Analogy: It's like flushing a toilet – you either push the handle enough to flush completely, or nothing happens. There's no "half-flush."
12. During muscle relaxation, what happens to Ca2+ in the sarcoplasm?
- It binds to RyRs, causing them to close.
- It is actively pumped back into the sarcoplasmic reticulum.
- It is released from troponin, and then diffuses out of the cell.
- It remains bound to troponin, keeping active sites exposed.
Correct (b): Relaxation requires active pumping of Ca2+ back into the SR by SERCA pumps, which lowers sarcoplasmic Ca2+ levels.
Incorrect: Ca2+ detaches from troponin when its concentration drops; it doesn't diffuse out of the cell; RyRs are closed by low Ca2+ (indirectly).
13. Which component of the thin filament directly binds to Ca2+ ions to initiate contraction?
- Actin
- Tropomyosin
- Troponin T
- Troponin C
Correct (d): Troponin C (TnC) is the specific subunit of the troponin complex that binds Ca2+ ions, initiating the conformational change leading to contraction.
Incorrect: Actin has myosin-binding sites; tropomyosin blocks them; TnT binds tropomyosin.
14. What happens to ADP and Pi immediately prior to the power stroke?
- Both ADP and Pi bind to the myosin head.
- Both ADP and Pi are released from the myosin head.
- Pi is released, while ADP remains bound.
- ADP is released, while Pi remains bound.
Correct (c): After the energized myosin head (with ADP + Pi) binds to actin, Pi is released, triggering the power stroke. ADP is released during the power stroke itself.
15. If a motor neuron's action potential fails to reach the presynaptic terminal, what is the direct consequence?
- Continuous muscle contraction due to uncontrolled ACh release.
- Increased sensitivity of the muscle to acetylcholine.
- No acetylcholine release, and thus no muscle contraction.
- Enhanced Ca2+ reuptake into the sarcoplasmic reticulum.
Correct (c): The action potential reaching the presynaptic terminal is the critical trigger for Ca2+ influx and subsequent ACh release. Without it, the NMJ process fails.
Incorrect: Without the AP, there's no release, controlled or uncontrolled. Receptor sensitivity isn't directly altered. Ca2+ reuptake is for relaxation, not relevant here.
16. The specialized endoplasmic reticulum that stores and releases Ca2+ ions in a muscle fiber is called the ____________________.
17. The functional contractile unit of a myofibril, extending from one Z-disc to the next, is the _________.
18. The release of _________ from the motor neuron terminal initiates the process at the neuromuscular junction.
19. During the cross-bridge cycle, the binding of new ATP to myosin causes it to _________ from actin.
20. DHPRs in T-tubules are mechanically linked to _________ on the SR, which act as Ca2+ release channels.
Quiz Complete!
Your Score:
0%
0 / 0 correct