Excitability: PHYSIOLOGY OF EXCITABLE TISSUES
Excitability
Excitability: The Ability to Respond and Communicate
Excitability refers to the ability of a cell to respond to a stimulus by generating an electrical signal called an action potential. It can be defined as a physical chemical change that occurs when a stimulus is applied on a tissue. A stimulus is an external agent that produces excitation in a tissue. This electrical signal is then propagated along the cell membrane or transmitted to other cells, leading to a specific physiological response.
The action potential is a transient, rapid, and self-propagating reversal of the electrical potential across the cell membrane. This electrical signal is the medium through which cells rapidly transmit information, either along the length of an individual cell or to other cells via specialized junctions. This property is crucial for rapid communication and coordination within the body, underpinning virtually every complex physiological function, from perception and thought to movement and visceral regulation.
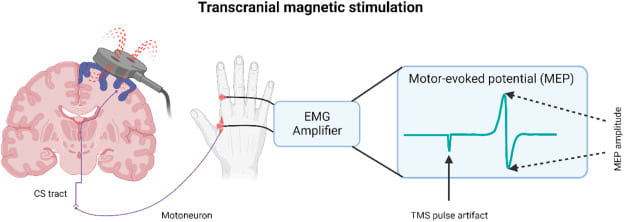
Analogy for Understanding: The Tripwire
Think of an excitable cell like a highly sensitive electrical tripwire or alarm system. The resting state is the armed system waiting for a trigger. The stimulus is the pressure that activates the tripwire. The action potential is the immediate, swift, and uniform "alarm bell" that rings loudly and clearly, sending its message through the system to orchestrate a coordinated response.
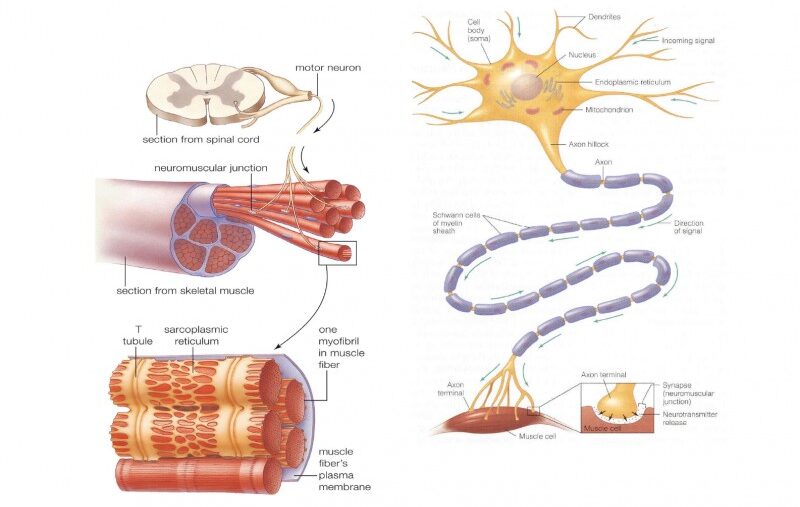
2. Excitable Cells
While all living cells exhibit some degree of responsiveness, only a select group possess the highly specialized machinery to generate and propagate rapid electrical signals. These are the "excitable cells."
Neurons (Nerve Cells): The Master Communicators
Expanded Role: Neurons are the fundamental units of the nervous system. Their primary function is the transmission of electrical and chemical signals for sensory input, integration, motor output, cognition, and emotion.
Unique Features: They possess specialized structures like dendrites (to receive signals), a cell body (soma), and a long axon (to transmit signals), often insulated by a myelin sheath to speed conduction.
Muscle Cells: The Effectors of Movement
Muscle cells are specialized for contraction, which generates force and movement. Their excitability is the prerequisite for this mechanical action.
Skeletal Muscle Cells:
Responsible for all voluntary movements (walking, speaking, breathing). When a motor neuron sends an action potential, it triggers a muscle action potential, leading to contraction.
Cardiac Muscle Cells:
Found only in the heart, responsible for the rhythmic and involuntary pumping of blood. They possess autorhythmicity and have distinctively long action potentials for coordinated contractions.
Smooth Muscle Cells:
Mediate involuntary movements in the walls of internal organs like the digestive tract, blood vessels, and urinary bladder. Their excitability is influenced by stretch, local chemicals, and the autonomic nervous system.
Glandular Cells: The Secretory Responders
Role Expansion: Many glandular cells (e.g., in the adrenal medulla, pancreas) exhibit excitability. They can respond to an electrical stimulus from a neuron by generating their own electrical event (depolarization or action potential).
Excitability Link: This electrical event is typically coupled to the release of their secretions (e.g., hormones, digestive enzymes). For example, adrenal medullary cells depolarize in response to a neuronal signal, triggering Ca²⁺ influx and the exocytosis of epinephrine. This ensures precise and rapid control over hormone release.
Membrane Potential
The capacity of these cells to generate electrical signals rests entirely on the idea of membrane potential.
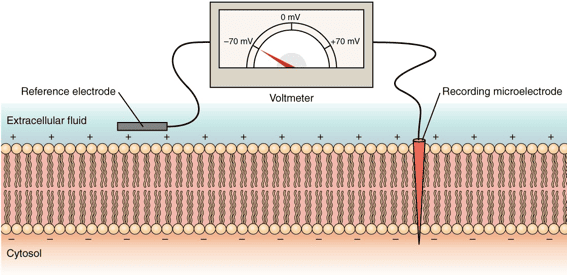
This is the voltage difference across the cell's outer boundary, a stored electrical energy created by an uneven distribution of ions (electrically charged particles) inside the cell (ICF) and outside the cell (ECF).
Resting Membrane Potential (RMP)
When an excitable cell is quiet, it maintains a stable, baseline electrical charge called the Resting Membrane Potential (RMP). In this state, the inside of the cell consistently holds a negative charge relative to the outside (e.g., -70 mV in neurons, -90 mV in skeletal muscle).
Creating and Maintaining the RMP
The RMP is a dynamic state, constantly maintained by an interplay of three factors:
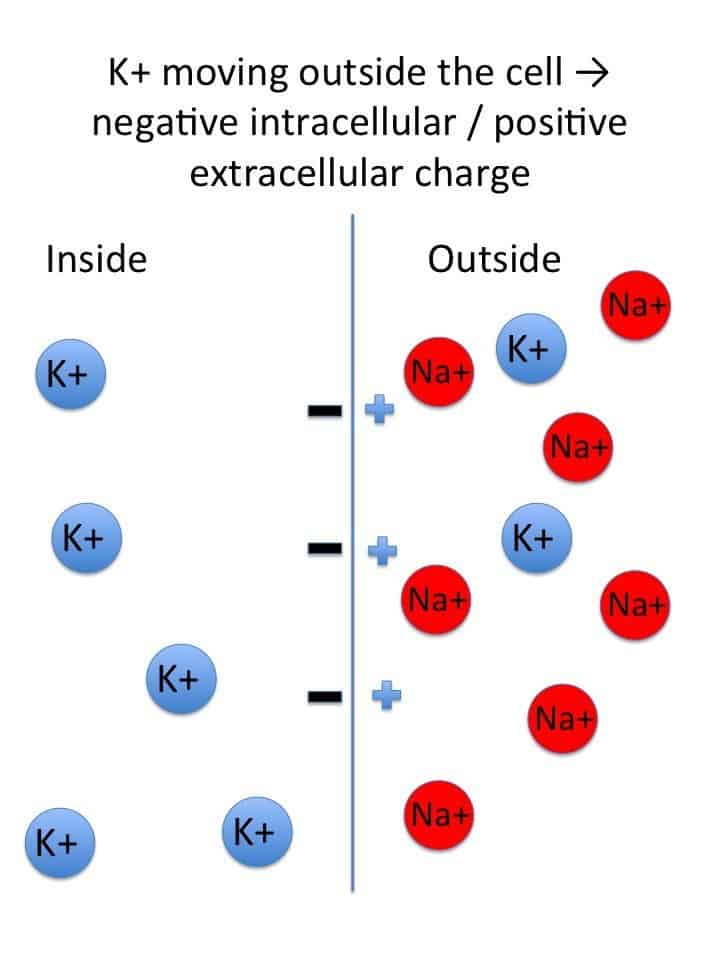
- Ion Gradients: The Concentration Divide
The foundation is the different concentrations of key ions: a high concentration of Na⁺ outside the cell and a high concentration of K⁺ inside the cell. - Selective Permeability: The Leaky Gates
At rest, the membrane is significantly more permeable to K⁺ than to Na⁺ because there are many more open K⁺ "leak" channels than Na⁺ leak channels. - Sodium-Potassium ATPase (Na⁺/K⁺-ATPase) Pump: The Gradient Upholder
This active transporter continually pumps 3 Na⁺ ions out for every 2 K⁺ ions it pumps in, directly maintaining the concentration gradients and contributing a small amount to the RMP's negativity (making it an electrogenic pump).
Equilibrium Potential (Nernst Potential)
The equilibrium potential for a specific ion is the membrane voltage at which there is no net movement of that ion across the membrane. At this voltage, the electrical force is perfectly balanced by the chemical (concentration) force. The Nernst Equation calculates this value:
E_ion = (RT / zF) * ln([ion]out / [ion]in)
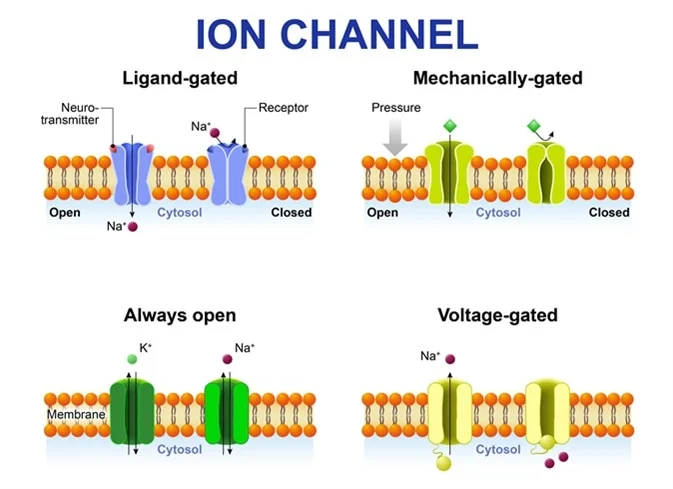
Ion Channels
These are specialized proteins that form pores for specific ions to cross the membrane.
Types Relevant to Excitability:
- Leak Channels: These channels are always open and are instrumental in establishing the RMP, particularly the K⁺ leak channels.
Gated Channels: The Responsive Switches
These channels open or close only in response to a particular trigger and are essential for generating action potentials.
Voltage-Gated Channels
Open or close in direct response to changes in membrane voltage. They are the key drivers of the action potential.
Ligand-Gated Channels (Chemically Gated)
Open or close when a specific chemical messenger (a ligand), such as a neurotransmitter, binds to them.
Mechanically Gated Channels
Open or close when they are physically deformed or stretched, critical for sensory perception like touch and pressure.
Initiating the Response: Stimulus and Threshold
The Stimulus: A Call to Action
A stimulus is any detectable change (electrical, chemical, or mechanical) in the cell's environment that has the potential to alter its RMP.
- Depolarization: A shift in membrane voltage where the inside of the cell becomes less negative (e.g., from -70 mV to -50 mV).
- Hyperpolarization: A shift where the inside of the cell becomes more negative (e.g., from -70 mV to -90 mV).
Threshold: The Point of No Return
Threshold is the crucial voltage level that depolarization must reach for an action potential to fire (typically around -55 mV in neurons). It is an "all-or-none" event: if a stimulus causes a depolarization that reaches threshold, a full action potential fires. If it does not, nothing happens.
The Action Potential
The action potential is the primary electrical signal employed by excitable cells to swiftly transmit information across significant distances. It stands as an "all-or-nothing" phenomenon: once initiated, it proceeds through its entire sequence with consistent strength, never diminishing.
Requirements for an Action Potential:
- Resting Membrane Potential (RMP): The cell needs a stable, negative baseline electrical charge.
- Voltage-Gated Ion Channels: These specialized channels respond specifically to changes in the membrane's electrical charge. The key players are:
- Voltage-Gated Sodium (Na⁺) Channels: Responsible for the rapid depolarization. They have a fast activation gate and a slower inactivation gate.
- Voltage-Gated Potassium (K⁺) Channels: Responsible for repolarization. They open more slowly in response to depolarization.
- Threshold Potential: A specific voltage level that must be reached for the action potential to be irrevocably triggered.
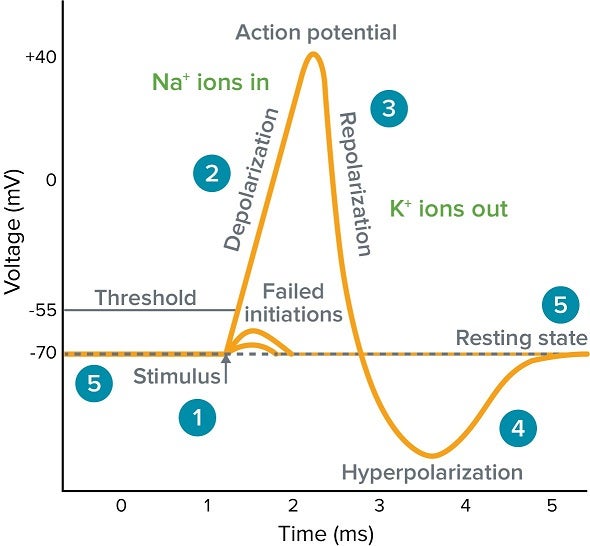
Stages of an Action Potential
1. Resting State (e.g., -70 mV)
All voltage-gated Na⁺ and K⁺ channels are closed. The RMP is maintained by K⁺ leak channels and the Na⁺/K⁺ pump.
2. Depolarization to Threshold (to -55 mV)
A local stimulus causes a few voltage-gated Na⁺ channels to open, allowing a small amount of Na⁺ to enter. If enough Na⁺ enters to raise the membrane potential to the threshold level, an action potential is triggered.
3. Rising Phase (Depolarization, to +30 mV)
Once threshold is reached, a vast number of voltage-gated Na⁺ channels open very rapidly. A massive and swift surge of Na⁺ into the cell causes the inside of the membrane to become positive.
4. Repolarization Phase (from +30 mV down)
At the peak, the voltage-gated Na⁺ channels inactivate (their inactivation gates close), stopping Na⁺ influx. Simultaneously, the slower voltage-gated K⁺ channels are now fully open, allowing a significant outflow of K⁺, which rapidly restores the membrane's negative charge.
5. Afterhyperpolarization (Undershoot)
The voltage-gated K⁺ channels close slowly, allowing K⁺ to continue exiting for a brief period. This causes the membrane to become temporarily more negative than the RMP.
6. Return to Rest
The slow K⁺ channels finally close, and the ever-active Na⁺/K⁺ pump helps to re-establish the original ion concentration gradients, returning the membrane to its stable RMP.
Defining Features of Action Potentials
- All-or-Nothing: If the threshold is crossed, the action potential unfolds completely with the same magnitude. If not, no action potential occurs.
- Non-Decremental: Action potentials are continuously re-generated along the membrane and do not lose strength as they move.
Refractory Periods
- Absolute Refractory Period: During the rising and peak phases, when Na⁺ channels are either open or inactivated, no second stimulus, regardless of its intensity, can trigger another action potential. This ensures one-way propagation of the signal.
- Relative Refractory Period: During the afterhyperpolarization phase, a stimulus that is stronger than normal can provoke another action potential.
Propagation of Action Potentials: Spreading the Message
The electrical shift at one point on the membrane triggers the opening of voltage-gated Na⁺ channels in the immediately adjacent area. This process repeats, moving the signal along the length of the nerve or muscle fiber.
Myelination (in Nerve Cells): Enhancing Speed
Many nerve fibers are insulated by a fatty myelin sheath. Action potentials therefore appear to "jump" from one uninsulated gap (a node of Ranvier) to the next. This rapid "jumping" process is termed saltatory conduction and dramatically increases the signal's speed.
Factors Influencing Conduction Speed:
- Fiber Diameter: Larger diameter fibers conduct signals more quickly.
- Myelination: Myelinated fibers transmit signals considerably faster than unmyelinated fibers.
Inhibition of Excitability
Just as cells must generate signals, they also need ways to inhibit them, ensuring precise control and preventing uncontrolled firing.
Hyperpolarization: Driving Further from Threshold
Inhibitory neurotransmitters (like GABA or glycine) open ion channels that either allow Cl⁻ to enter the cell or K⁺ to leave. The outcome is an increase in the negative charge inside the cell (e.g., from -70 mV to -75 mV), making it significantly harder for the cell to reach the threshold and fire an action potential.
Presynaptic Inhibition: Muting the Signal at its Source
An inhibitory neuron releases neurotransmitter (e.g., GABA) directly onto the axon terminal of an excitatory neuron. This reduces the electrical charge of the terminal, so when an action potential arrives, fewer excitatory neurotransmitters are released. This allows for fine-tuning and selective reduction of specific signals.
Pharmacological Inhibition: Manipulating Channels
A vast array of drugs and toxins work by directly interfering with ion channels.
- Local Anesthetics (e.g., Lidocaine): Block voltage-gated Na⁺ channels, preventing action potentials in pain-sensing nerves.
- Tetrodotoxin (TTX, from pufferfish): A potent blocker of voltage-gated Na⁺ channels, causing paralysis.
- Anti-epileptic Drugs: Some work by enhancing GABA's inhibitory effects or stabilizing Na⁺ channels to prevent excessive firing.
Clinical Significance of Excitability
An in-depth comprehension of cellular excitability is absolutely vital for understanding, diagnosing, and creating effective treatments for numerous conditions affecting the nervous system and muscles.
Conditions of the Nervous System and Muscles:
- Epilepsy: Marked by episodes of abnormal, synchronized, and excessive electrical firing of large groups of neurons in the brain, resulting in seizures.
- Multiple Sclerosis (MS): An autoimmune disease where the myelin sheath insulating nerve fibers is destroyed. This slows, weakens, or completely blocks action potential propagation, leading to muscle weakness and sensory disturbances.
- Myasthenia Gravis: An autoimmune disease that destroys acetylcholine receptors at the neuromuscular junction, reducing the ability of nerve signals to excite muscle cells and leading to muscle weakness.
- Cardiac Arrhythmias: Irregular heart rhythms stemming from abnormalities in the electrical excitability of heart muscle cells, leading to potentially life-threatening disruptions to the heart's pumping action.
Electrolyte Imbalances
The precise balance of ions is paramount for proper excitability.
Hyperkalemia (Elevated K⁺)High extracellular K⁺ makes the resting membrane potential less negative (closer to threshold). While this might initially increase excitability, prolonged depolarization can inactivate voltage-gated Na⁺ channels, rendering cells inexcitable. This is life-threatening for heart muscle cells and can lead to cardiac arrest.
Hypokalemia (Low K⁺)Low extracellular K⁺ makes the resting membrane potential more negative (hyperpolarized). This moves the cell further from threshold, making it less excitable and leading to symptoms like muscle weakness and dangerous heart arrhythmias.
Sodium Imbalances (Hypernatremia/Hyponatremia)Since the influx of Na⁺ is the primary driver of depolarization, imbalances in Na⁺ levels can significantly impair the ability of nerve and muscle cells to generate action potentials.
Calcium Imbalances- Hypocalcemia (Low Ca²⁺): Low extracellular calcium paradoxically increases nerve cell excitability by making voltage-gated Na⁺ channels open more easily. This can lead to muscle spasms (tetany).
- Hypercalcemia (High Ca²⁺): High extracellular calcium stabilizes Na⁺ channels, making them harder to open. This decreases neuronal excitability, potentially leading to muscle weakness and reduced neurological function.
Test Your Knowledge
An Excitability Exam covering core neurophysiology concepts.
1. Which ion is primarily responsible for the rapid depolarization (rising phase) of a typical neuronal action potential?
- Potassium (K+)
- Chloride (Cl-)
- Sodium (Na+)
- Calcium (Ca2+)
Correct (c): The rapid influx of positively charged Na+ ions through voltage-gated Na+ channels causes the membrane potential to swiftly become positive during the rising phase.
Incorrect: K+ is for repolarization, Cl- for inhibition, and Ca2+ for neurotransmitter release.
Analogy: Think of Na+ as the "gas pedal" for the action potential. Pushing it hard (opening Na+ channels) makes the electrical signal quickly accelerate upwards.
2. The Resting Membrane Potential (RMP) is primarily maintained by which two factors?
- Voltage-gated Na+ channels and Na+/K+-ATPase pump
- Leak K+ channels and voltage-gated K+ channels
- Leak K+ channels and Na+/K+-ATPase pump
- Ligand-gated channels and voltage-gated Na+ channels
Correct (c): The RMP is established by the Na+/K+-ATPase pump (which creates the gradients) and the high permeability of the membrane to K+ ions through K+ leak channels (allowing K+ to slowly exit).
Incorrect: Voltage-gated and ligand-gated channels are primarily involved in generating signals (action potentials, synaptic potentials), not maintaining the baseline RMP.
3. What event immediately follows the membrane potential reaching threshold?
- Voltage-gated K+ channels rapidly open.
- Voltage-gated Na+ channels rapidly open in a positive feedback loop.
- The Na+/K+-ATPase pump becomes more active.
- The cell hyperpolarizes due to Cl- influx.
Correct (b): Reaching threshold triggers a massive opening of voltage-gated Na+ channels, leading to a huge Na+ influx and the rapid depolarization. This is a positive feedback loop.
Incorrect (a): K+ channels open slowly and are for repolarization.
Analogy: Reaching threshold is like the first domino falling, triggering a chain reaction where all the other dominoes (voltage-gated Na+ channels) quickly topple over.
4. The absolute refractory period of an action potential is primarily caused by:
- The slow closing of voltage-gated K+ channels.
- The inactivation of voltage-gated Na+ channels.
- The continued activity of the Na+/K+-ATPase pump.
- The binding of inhibitory neurotransmitters.
Correct (b): During this period, the voltage-gated Na+ channels are in an inactivated state and cannot open again, regardless of stimulus strength, preventing another action potential.
Incorrect (a): Slow closing of K+ channels contributes to the relative refractory period.
Analogy: The inactivation gate of the Na+ channel is like a "do not disturb" sign. Once it's up, no matter how hard you knock, you can't start another action potential until it's taken down.
5. Myelination of an axon primarily serves to:
- Increase the amplitude of action potentials.
- Slow down the conduction velocity of action potentials.
- Prevent the action potential from going backward.
- Increase the conduction velocity of action potentials.
Correct (d): Myelin acts as an electrical insulator, forcing the action potential to "jump" between nodes of Ranvier (saltatory conduction), which significantly speeds up signal transmission.
Incorrect (a): Action potential amplitude is "all-or-nothing."
Incorrect (c): The refractory period ensures unidirectional propagation.
6. Which condition would make a cell less excitable by hyperpolarizing its RMP?
- Opening of voltage-gated Na+ channels.
- Decreased K+ leak channels activity.
- Increased Cl- influx through ligand-gated channels.
- Reduced activity of the Na+/K+-ATPase pump.
Correct (c): If negative Cl- ions enter the cell, they make the inside more negative, driving the membrane potential further away from the threshold, thus reducing excitability.
Incorrect (a): Opening Na+ channels causes depolarization, making it more excitable.
7. In the context of action potentials, "all-or-nothing" means:
- The cell either fires an action potential, or it dies.
- All ion channels open simultaneously or none do.
- Once threshold is reached, a full-sized action potential fires, or none fires at all.
- All parts of the cell depolarize at the exact same time.
Correct (c): If a stimulus is strong enough to reach threshold, a full-sized action potential occurs. If it's below threshold, no action potential occurs. The size of the AP is independent of stimulus strength.
Analogy: It's like flipping a light switch. You either press it hard enough to turn the light completely ON, or it stays OFF. There's no "half-on" setting.
8. Which phase is characterized by K+ outflow and Na+ channel inactivation?
- Resting state
- Depolarization to threshold
- Rising phase
- Repolarization phase
Correct (d): During repolarization, voltage-gated Na+ channels inactivate (stop Na+ influx), and voltage-gated K+ channels are fully open, allowing K+ to exit the cell, bringing the membrane potential back down.
9. A drug that blocks voltage-gated Na+ channels would primarily affect:
- Maintaining the resting membrane potential.
- The ability to generate action potentials.
- The rate of neurotransmitter release.
- The speed of K+ efflux during repolarization.
Correct (b): Voltage-gated Na+ channels are essential for the rapid depolarization phase. Blocking them prevents the action potential from initiating and propagating.
Analogy: Blocking Na+ channels is like taking the ignition key out of a car. You can't start the engine (action potential) at all.
10. Which of the following best describes Multiple Sclerosis (MS)?
- Hyperexcitability of neurons leading to seizures.
- Impaired action potential conduction due to demyelination.
- Reduced ability of neurotransmitters to excite muscle cells.
- Abnormalities in cardiac muscle excitability.
Correct (b): MS is characterized by the destruction of the myelin sheath that insulates axons, which directly disrupts the efficient and rapid propagation of action potentials.
Incorrect (a): This describes epilepsy.
Incorrect (c): This describes Myasthenia Gravis.
11. The Equilibrium Potential for an ion is the membrane potential where:
- The concentration gradient for that ion is zero.
- There is no net movement of that ion across the membrane.
- All channels for that ion are closed.
- The Na+/K+-ATPase pump stops working for that ion.
Correct (b): At the equilibrium potential, the electrical force pulling the ion is exactly equal and opposite to the chemical (concentration) force pushing it, resulting in no net movement.
12. Presynaptic inhibition reduces an excitatory signal by:
- Causing the postsynaptic neuron to hyperpolarize.
- Directly blocking the excitatory neurotransmitter.
- Reducing neurotransmitter release from the presynaptic terminal.
- Increasing the reuptake of excitatory neurotransmitter.
Correct (c): Presynaptic inhibition involves an inhibitory neuron acting on the axon terminal of an excitatory neuron, reducing the amount of neurotransmitter released when an action potential arrives.
Incorrect (a): This would be postsynaptic inhibition.
13. A patient with hypokalemia (low extracellular K+) would likely experience:
- Increased neuronal excitability, leading to seizures.
- Hyperpolarization of the RMP, leading to muscle weakness.
- Rapid depolarization of cardiac cells, causing arrhythmias.
- Enhanced neurotransmitter release due to increased Ca2+ influx.
Correct (b): With less K+ outside, the K+ gradient out of the cell becomes steeper, causing more K+ to leave. This makes the inside more negative (hyperpolarized), moving the RMP further from threshold and making cells less excitable.
14. What is the role of the inactivation gate of the voltage-gated Na+ channel?
- To open rapidly at threshold to initiate depolarization.
- To close slowly to ensure prolonged Na+ influx.
- To close, terminating Na+ influx and causing the refractory period.
- To allow K+ to exit the cell during repolarization.
Correct (c): The inactivation gate closes a few milliseconds after the activation gate opens, stopping Na+ influx. This is essential for repolarization and prevents immediate re-firing (absolute refractory period).
Incorrect (a): This is the role of the activation gate.
15. Which ion's movement is primarily responsible for the "afterhyperpolarization" (undershoot) phase?
- Rapid Na+ influx
- Continued K+ efflux
- Cl- influx
- Ca2+ influx
Correct (b): Afterhyperpolarization occurs because voltage-gated K+ channels are slow to close, allowing K+ to continue exiting the cell for a short period, making the membrane temporarily more negative than RMP.
16. The critical electrical level that must be reached for an action potential to be generated is known as the _________ potential.
17. Local anesthetics like Lidocaine work by blocking voltage-gated _________ channels.
18. In Multiple Sclerosis, the loss of the myelin sheath leads to impaired action potential _________.
19. The period when a second AP cannot be generated, regardless of stimulus strength, is the _________ refractory period.
20. Neurotransmitters like GABA and glycine can inhibit excitability by causing the influx of _________ ions.
Quiz Complete!
Your Score:
0%
0 / 0 correct