Common Abnormalities: Teratology and Teratogenesis
Common Abnormalities: Teratology and Teratogenesis
1. Teratology
Teratology is the scientific study of abnormal physiological development, specifically focusing on the causes, mechanisms, and patterns of birth defects, also known as congenital malformations. The term comes from the Greek "teras," meaning monster or marvel.
Key Concepts in Teratology
Congenital Malformations (Birth Defects) are structural, functional, or metabolic abnormalities present at birth. These can range from minor cosmetic issues to severe, life-threatening conditions. Not all congenital conditions are visible at birth (e.g., some heart defects or metabolic disorders). They are classified into several distinct categories based on their origin.
Malformation
A primary structural defect resulting from an intrinsically abnormal developmental process. The blueprint itself was flawed from the beginning.
Example: Polydactyly (extra fingers/toes), Spina Bifida.
Disruption
A defect resulting from the extrinsic breakdown of, or interference with, an originally normal developmental process. The blueprint was normal, but something damaged the structure as it was forming.
Example: Limb amputation due to amniotic bands wrapping around it.
Deformation
An abnormal form, shape, or position of a body part caused by extrinsic mechanical forces acting on a normally developed structure.
Example: Clubfoot due to intrauterine crowding, limiting space for the feet to grow properly.
Dysplasia
An abnormal organization of cells into tissues. The problem lies in how the cells themselves are structured and arranged.
Example: Skeletal dysplasias like achondroplasia (a form of dwarfism).
Syndrome
A group of anomalies that occur together and have a specific, common, known cause.
Example: Down syndrome (caused by Trisomy 21), Fetal Alcohol Syndrome.
Association
A non-random occurrence of two or more anomalies that appear together more often than by chance, but for which a common cause has not yet been identified.
Example: VACTERL association (Vertebral, Anal, Cardiac, Tracheo-Esophageal, Renal, Limb defects).
Factors Contributing to Birth Defects
While teratogens are a major focus, it's important to understand the broader categories of factors that can lead to congenital malformations.
2. Teratogenesis
Teratogenesis is the process by which a teratogen (an agent that causes birth defects) acts on an embryo or fetus to produce a congenital malformation. The study of this process is governed by a set of foundational concepts known as Wilson's Principles.
Principle 1: Susceptibility (Genotype)
The genetic makeup of the embryo and mother determines their susceptibility to a teratogen. What harms one individual may have no effect on another due to genetic differences in metabolism and cellular repair.
Principle 2: Dosage & Duration
The amount of the teratogen and the length of exposure are critical. Generally, a higher dose or a longer duration of exposure increases the risk and severity of the resulting defect.
Principle 3: Timing of Exposure (Critical Periods)
This is arguably the most crucial principle. The susceptibility of an organ system to a teratogen varies dramatically with its stage of development.
Pre-implantation Period (Weeks 1-2)
The "all-or-nothing" period. Exposure to a teratogen usually results in either the death of the embryo or its complete recovery with no defects, as the cells are still totipotent and can be replaced.
Embryonic Period (Weeks 3-8)
The most sensitive period for major malformations. This is when organogenesis occurs, and each organ system has its own critical window of vulnerability (e.g., heart: weeks 3-5; CNS: weeks 3-16+).
Fetal Period (Weeks 9 to Birth)
Exposure during this period generally does not cause major structural defects but can lead to functional problems, growth retardation, and minor abnormalities, especially in the still-developing brain.
Principle 4: Mechanisms
Teratogens exert their effects through specific cellular and molecular mechanisms, such as interfering with cell proliferation or migration, inducing cell death (apoptosis), or disrupting biochemical pathways.
Principle 5: Manifestations
The final outcome of teratogenic exposure can be one of four manifestations: death, malformation, growth retardation, or functional deficit.
Classes of Teratogens
Teratogens are substances that can cause birth defects when a fetus is exposed during pregnancy. They can be broadly categorized into several classes, each with well-documented examples and associated defects. The risk and severity of abnormalities depend on the type of agent, timing, dosage, and duration of exposure.
Infectious Agents (TORCH Infections)
The acronym TORCH helps remember some of the most well-known infectious teratogens:
- Toxoplasmosis: A parasitic infection that can cause hydrocephalus and intracranial calcifications.
- Others (e.g., Syphilis, Varicella-Zoster, Zika, Parvovirus B19): Zika is known for causing microcephaly, while syphilis can lead to congenital deafness and other issues.
- Rubella (German measles): Can result in a classic triad of cataracts, cardiac malformations, and deafness.
- Cytomegalovirus (CMV): A common virus that can cause microcephaly, hearing loss, and intellectual disability.
- Herpes Simplex Virus: Can lead to skin lesions, microcephaly, and eye problems.
Drugs and Chemicals
- Thalidomide: A classic example that caused severe limb reduction defects (phocomelia).
- Alcohol (Ethanol): The leading preventable cause of non-genetic birth defects, leading to Fetal Alcohol Syndrome (FAS) with distinct facial anomalies, growth retardation, and CNS dysfunction.
- Tobacco & Nicotine: Smoking is associated with low birth weight, premature delivery, and can affect the development of the fetal brain and lungs.
- Retinoids (e.g., Isotretinoin/Accutane): Highly teratogenic, causing severe CNS, facial, cardiac, and ear malformations.
- Anticonvulsants (e.g., Valproic Acid, Phenytoin): Associated with neural tube defects, cleft lip/palate, and cardiac defects.
- ACE Inhibitors: Can cause renal failure and oligohydramnios (insufficient amniotic fluid).
- Warfarin: An anticoagulant that can cause skeletal abnormalities, including chondrodysplasia punctata.
- Certain Antibiotics (e.g., Tetracycline): Can cause yellow staining of teeth and affect long bone growth.
- Recreational Drugs (e.g., Cocaine, Heroin): Can lead to low birth weight, withdrawal symptoms in the newborn, and learning or behavioral problems.
Environmental Toxins
- Heavy Metals (e.g., Mercury, Lead): Can cause significant CNS damage and developmental delays. Mercury is often found in certain types of fish, and lead can be in old paint and pipes.
- Polychlorinated Biphenyls (PCBs): Industrial chemicals that can lead to developmental and neurological problems.
- Herbicides and Industrial Solvents: Exposure to certain chemicals used in agriculture and manufacturing can be harmful.
Physical Agents
- Ionizing Radiation (e.g., X-rays, Radiation Therapy): High doses can cause microcephaly, intellectual disability, and growth restriction.
- Hyperthermia: Prolonged high body temperature from fever or use of hot tubs and saunas in early pregnancy can increase the risk for neural tube defects.
Maternal Factors & Metabolic Conditions
- Maternal Diabetes Mellitus (poorly controlled): High blood sugar levels can increase the risk of cardiac defects, neural tube defects, and caudal regression syndrome.
- Maternal Phenylketonuria (PKU): Uncontrolled high phenylalanine levels are highly teratogenic to the fetal brain.
- Maternal Obesity: Associated with a higher risk of neural tube defects and cardiac anomalies.
- Nutritional Deficiencies: Folic acid deficiency is a major, preventable cause of neural tube defects like spina bifida.
- Autoimmune Diseases (e.g., Lupus): The condition itself or the medications used for treatment can pose risks.
Prevention and Management
While not all birth defects are preventable, proactive measures can significantly reduce their incidence and impact.
- Preconception Counseling: Discussing risks like chronic health conditions and medications with a healthcare provider before becoming pregnant is ideal.
- Folic Acid Supplementation: Taking a daily prenatal vitamin with at least 400 micrograms of folic acid is a highly effective measure for preventing neural tube defects.
- Avoidance of Known Teratogens: This includes abstaining from alcohol, smoking, and recreational drugs, as well as being cautious with medications and chemical exposures.
- Vaccinations: Ensuring immunizations, such as for rubella, are up to date can prevent congenital infections.
- Regular Prenatal Care: Utilizing prenatal tools like ultrasounds and maternal serum screening helps in the early identification of potential issues.
- Managing Health Conditions: Actively managing chronic conditions like diabetes or thyroid issues is crucial during pregnancy.
Chromosomal Abnormalities
These abnormalities result from errors in chromosome number (aneuploidy) or structure. They are often severe and can affect multiple organ systems, leading to distinct syndromes.
A. Aneuploidies (Abnormal Number of Chromosomes)
Aneuploidies are typically caused by non-disjunction—the failure of chromosomes to separate properly during meiosis.

Down Syndrome (Trisomy 21)
Cause: An extra copy of chromosome 21 (47, XX/XY, +21).
Incidence: ~1 in 700 live births; risk increases with maternal age.
Key Features:
- Characteristic Facial Features: Upward slanting eyes, epicanthal folds, flat nasal bridge.
- Intellectual Disability: Mild to moderate severity.
- Congenital Heart Defects: Very common (e.g., AV septal defect).
- Other Signs: Hypotonia (poor muscle tone), single palmar crease, increased risk of leukemia and early-onset Alzheimer's.
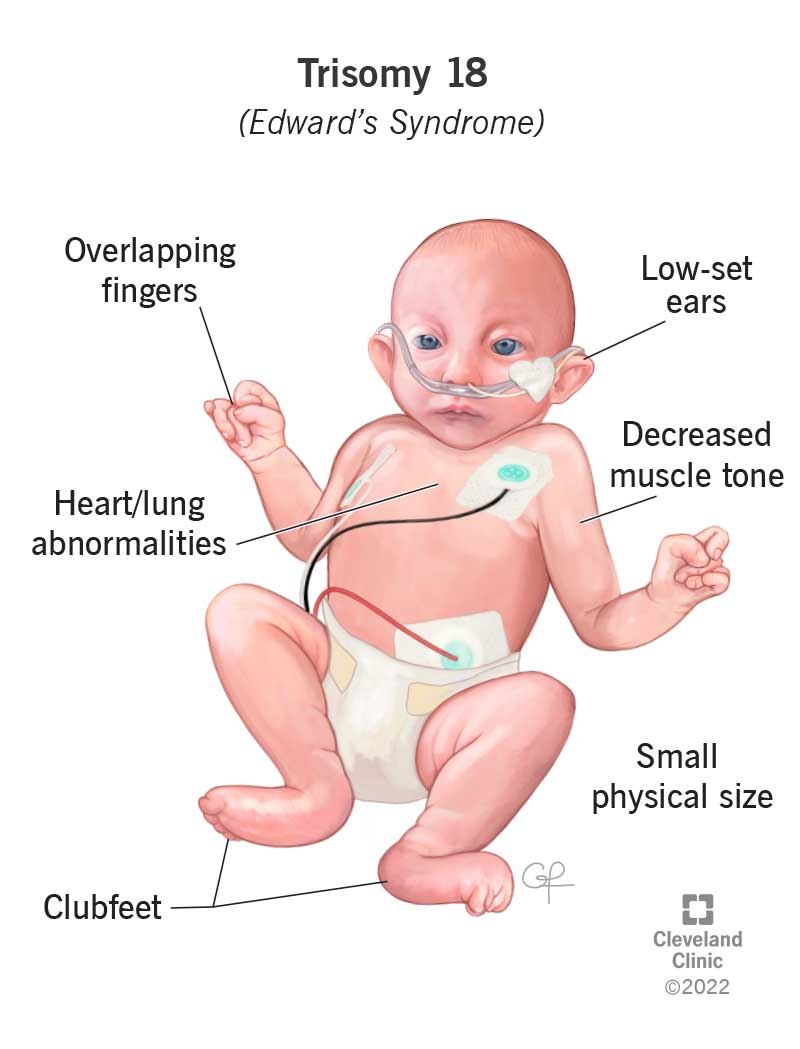
Edward Syndrome (Trisomy 18)
Cause: An extra copy of chromosome 18 (47, XX/XY, +18).
Incidence: ~1 in 5,000 live births; severe prognosis.
Key Features:
- Severe Intellectual Disability & Growth Retardation.
- Characteristic Physical Features: Small head (microcephaly), small jaw (micrognathia), low-set ears, clenched hands with overlapping fingers, rocker-bottom feet.
- Major Organ Defects: Severe heart and kidney malformations.
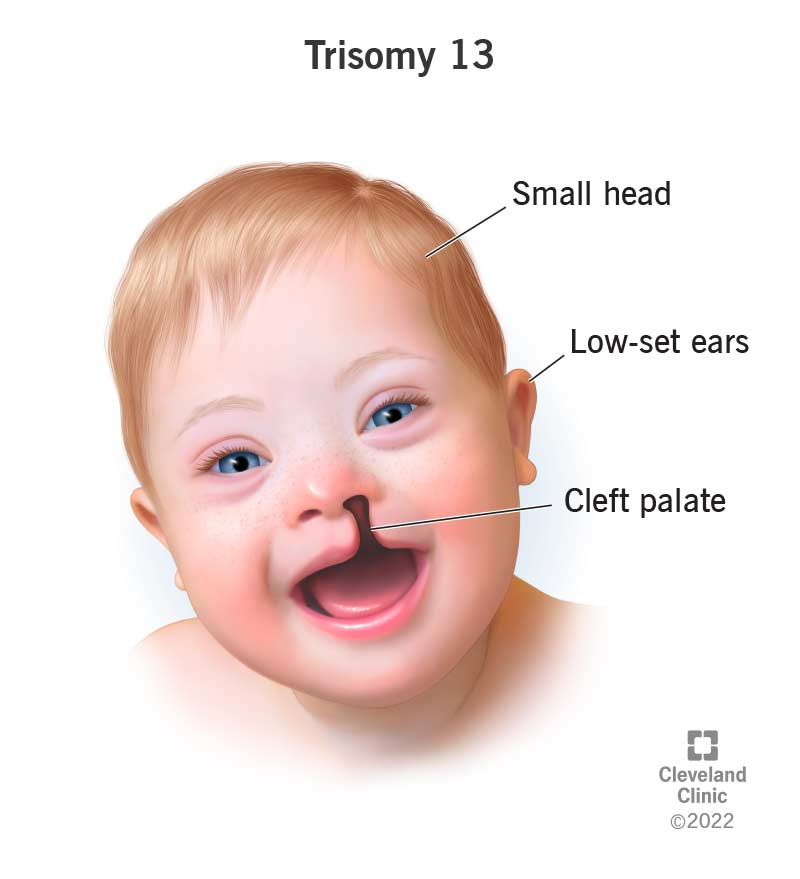
Patau Syndrome (Trisomy 13)
Cause: An extra copy of chromosome 13 (47, XX/XY, +13).
Incidence: ~1 in 16,000 live births; severe prognosis.
Key Features:
- Major CNS Malformations: Holoprosencephaly (failure of forebrain to divide).
- Facial Anomalies: Cleft lip and/or palate, small or absent eyes.
- Polydactyly (extra fingers or toes).
- Severe heart and renal defects.

Turner Syndrome (Monosomy X)
Cause: Affects females; absence of one X chromosome (45, XO).
Incidence: ~1 in 2,500 live female births.
Key Features:
- Short Stature and Webbed Neck.
- Ovarian Dysgenesis: Underdeveloped ovaries leading to infertility.
- Broad Chest with widely spaced nipples.
- Heart Defects: Coarctation of the aorta is common.
- Normal intelligence, but may have specific spatial learning difficulties.

Klinefelter Syndrome
Cause: Affects males; an extra X chromosome (47, XXY).
Incidence: ~1 in 500-1,000 live male births.
Key Features:
- Tall Stature.
- Hypogonadism: Small testes, leading to infertility and reduced testosterone.
- Gynecomastia (breast development).
- Increased risk of learning difficulties (often language-based).
- Frequently undiagnosed until puberty or an infertility workup.
B. Structural Chromosomal Abnormalities
These involve changes in the structure of a chromosome, such as deletions, duplications, or translocations. An important example is:
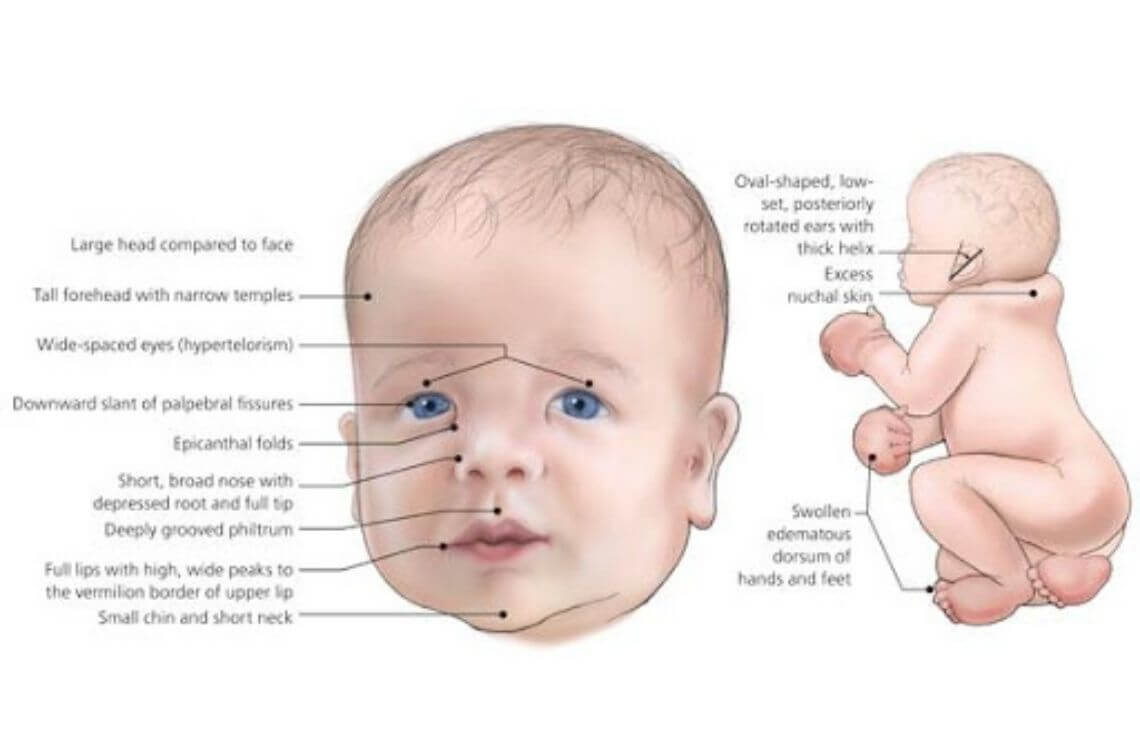
Cri-du-chat Syndrome
Caused by a deletion on the short arm of chromosome 5. It is characterized by severe intellectual disability, microcephaly, and a distinctive high-pitched, cat-like cry in infancy.
2. Single-Gene (Monogenic) Disorders
These disorders result from a mutation in a single gene and typically follow predictable Mendelian patterns of inheritance.
A. Autosomal Dominant Conditions
Only one copy of the mutated gene (from either parent) is needed to express the disease.
Achondroplasia
Cause: Mutation in the FGFR3 gene.
Key Features: The most common cause of dwarfism (short-limbed), characterized by a large head (macrocephaly) and prominent forehead. Intelligence is typically normal.
Marfan Syndrome
Cause: Mutation in the FBN1 gene (codes for fibrillin-1, a key connective tissue protein).
Key Features: Affects connective tissue. Leads to tall stature, long limbs, hypermobile joints, and severe cardiovascular complications (aortic aneurysm).
Huntington's Disease
Cause: Expansion of a CAG trinucleotide repeat in the HTT gene.
Key Features: A progressive neurodegenerative disorder with motor, cognitive, and psychiatric symptoms, typically with an onset in mid-adulthood.
B. Autosomal Recessive Conditions
Two copies of the mutated gene (one from each carrier parent) are needed to express the disease.
Cystic Fibrosis (CF)
Cause: Mutation in the CFTR gene, affecting chloride channels.
Key Features: Production of thick, sticky mucus that primarily damages the lungs and pancreas, leading to chronic respiratory infections and malabsorption.
Phenylketonuria (PKU)
Cause: Mutation in the PAH gene, leading to an enzyme deficiency.
Key Features: Inability to metabolize the amino acid phenylalanine. If untreated, it leads to severe intellectual disability. Managed by a strict diet and detectable by newborn screening.
Sickle Cell Anemia
Cause: Point mutation in the beta-globin gene (HbS).
Key Features: Red blood cells become sickle-shaped, causing painful vaso-occlusive crises, anemia, and organ damage. Common in populations from malaria-endemic regions.
C. X-Linked Recessive Conditions
Caused by a mutation on the X chromosome. Primarily affects males, as they have only one X chromosome. Females with one mutated copy are typically carriers.
Duchenne Muscular Dystrophy (DMD)
Cause: Mutation in the DMD gene, leading to an absence of the dystrophin protein.
Key Features: Progressive muscle wasting and weakness, leading to loss of ambulation in early teens and eventual respiratory and cardiac failure.
Hemophilia A and B
Cause: Deficiency of clotting Factor VIII (Hemophilia A) or Factor IX (Hemophilia B).
Key Features: Impaired blood clotting, leading to spontaneous or prolonged bleeding, especially into joints and muscles.
3. Multi-factorial & Environmental Abnormalities
This complex category includes some of the most common birth defects. They are caused by an intricate interplay of multiple genes and environmental factors, or are the direct result of a specific environmental exposure (teratogen).
A. Neural Tube Defects (NTDs)
Malformations of the brain or spinal cord from incomplete neural tube closure during weeks 3-4.
Common Types:
- Spina Bifida: Incomplete closure of the vertebral column, potentially exposing the spinal cord and causing paralysis or bladder/bowel dysfunction.
- Anencephaly: Failure of the cranial end to close, resulting in the absence of a major portion of the brain and skull. This condition is always lethal.
Prevention: Periconceptional folic acid supplementation significantly reduces the risk.
B. Cleft Lip and/or Palate
A congenital split in the upper lip and/or the roof of the mouth (palate).
Causes & Impact:
Caused by a mix of genes and environmental factors (e.g., smoking, certain medications). It can lead to difficulties with feeding, speech, and dental development, and requires surgical repair.
C. Congenital Heart Defects (CHDs)
The most common type of birth defect, involving abnormalities in the heart's structure.
Common Types:
- Ventricular Septal Defect (VSD): A hole between the lower chambers.
- Atrial Septal Defect (ASD): A hole between the upper chambers.
- Patent Ductus Arteriosus (PDA): Failure of a fetal blood vessel to close after birth.
- Tetralogy of Fallot: A complex defect involving four distinct abnormalities.
D. Fetal Alcohol Syndrome (FAS)
Caused by maternal alcohol consumption during pregnancy.
Key Features:
- Facial Anomalies: Short palpebral fissures, a thin upper lip, and a smooth philtrum.
- Growth Retardation.
- Central Nervous System Abnormalities: Intellectual disability and behavioral problems.
Prevention: Complete abstinence from alcohol during pregnancy.
E. Congenital Rubella Syndrome (CRS)
Caused by maternal infection with the rubella virus during early pregnancy.
Key Features (Classic Triad):
- Ocular Defects: Cataracts.
- Cardiac Defects: Patent ductus arteriosus (PDA).
- Sensorineural Deafness.
Prevention: MMR vaccination of women prior to pregnancy.
Test Your Knowledge
Check your understanding of the concepts covered in this post.
1. Which of the following terms describes a birth defect resulting from an extrinsically caused breakdown of an originally normal developmental process, such as limb amputation due to amniotic bands?
- Malformation
- Deformation
- Dysplasia
- Disruption
2. During which period of embryonic/fetal development is the conceptus most sensitive to major structural malformations due to teratogen exposure?
- Pre-implantation Period (Weeks 1-2)
- Embryonic Period (Weeks 3-8)
- Fetal Period (Weeks 9 to birth)
- Post-natal Period
3. According to Wilson's Principles of Teratogenesis, what is the most crucial factor determining the susceptibility of an organ system to a teratogen?
- The mother's age
- The duration of exposure
- The timing of exposure (Critical Periods)
- The father's genetic makeup
4. A significant proportion (40-50%) of birth defects fall into which causative category?
- Genetic Factors
- Environmental Factors
- Multi-factorial Inheritance
- Unknown Causes
5. Which teratogen is the leading preventable cause of birth defects, characterized by facial anomalies, growth retardation, and CNS dysfunction?
- Thalidomide
- Isotretinoin
- Alcohol (Ethanol)
- Valproic Acid
6. A group of anomalies occurring together that have a common cause is best described as a:
- Association
- Syndrome
- Malformation
- Deformation
7. Exposure to a teratogen during the "all or nothing" period (Weeks 1-2 post-fertilization) most commonly results in which outcome?
- Major structural malformations
- Functional deficits and growth retardation
- Either embryonic death or complete recovery without defects
- Minor cosmetic issues
8. Which of the following maternal factors/metabolic conditions is strongly associated with an increased risk of neural tube defects if poorly controlled?
- Maternal Phenylketonuria (PKU)
- Maternal Diabetes Mellitus
- Maternal Obesity
- All of the above
9. Which infectious agent is known to cause microcephaly and is primarily associated with the Zika virus?
- Toxoplasmosis
- Rubella
- Cytomegalovirus
- Zika virus
10. The classic triad of cataracts, cardiac malformations, and deafness is associated with maternal exposure to which teratogenic infection?
- Cytomegalovirus (CMV)
- Rubella
- Toxoplasmosis
- Herpes Simplex Virus
11. The scientific study of abnormal physiological development, focusing on the causes and patterns of birth defects, is known as ______________.
12. The process by which an agent acts on an embryo or fetus to produce a congenital malformation is called ______________.
13. ______________ is a birth defect caused by extrinsic mechanical forces, such as clubfoot due to intrauterine crowding.
14. The period during which organogenesis occurs (Weeks 3-8 post-fertilization) is also known as the ______________ period.
15. ______________ supplementation is crucial for preventing neural tube defects.
Quiz Complete!
Your Score:
0%
0 / 0 correct